
4-Hydroxycoumarins

4-Hydroxycoumarins belong to a class of vitamin K antagonist (VKA) anticoagulant drug molecules derived from coumarin by adding a hydroxy group at the 4 position to obtain 4-hydroxycoumarin, then adding a large aromatic substituent at the 3-position (the ring-carbon between the hydroxyl and the carbonyl). The large 3-position substituent is required for anticoagulant activity.
The primary mechanism of the 4-hydroxycoumarin drugs is the inhibition of vitamin K epoxide reductase. These compounds are not direct antagonists (in the pharmaceutical sense) of vitamin K, but rather act to deplete reduced vitamin K in tissues. For this reason vitamin K antagonizes their effect, and this has led to the loose terminology of vitamin K antagonism.
Origin
Although 4-hydroxycoumarin itself is not an anticoagulant, it is an important fungal metabolite from the precursor coumarin, which is also not an anticoagulant, and its production leads to further fermentative production of the natural anticoagulant dicoumarol. This happens in the presence of naturally occurring formaldehyde, which allows attachment of a second 4-hydroxycoumarin molecule through the linking carbon of the formaldehyde, to the 3-position of the first 4-hydroxycoumarin molecule, giving the semi-dimer the motif of the drug class. Dicoumarol appears in spoiled sweet clover silages and is considered a natural chemical substance of combined plant and fungal origin; mycotoxin also shares these properties. The identification of Dicoumarol in 1940 is the precursor of the drug class known as 4-Hydroxycoumarin. See warfarin for this history.
Effect
The synthetic drugs in the 4-hydroxycoumarin class are all noted primarily for their use as anticoagulants, though they can have several additional effects. All affect the normal metabolism of vitamin K in the body by inhibiting the enzyme vitamin K epoxide reductase which recycles vitamin K to active form. As such, these compounds form the most important and widely used subset of vitamin K antagonist drugs, but other such drugs exist which do not have the 4-hydroxycoumarin structure. All the vitamin K antagonist agents diminish the amount of available vitamin K in the body, and thus inhibit the action of vitamin K-dependent enzymes that are critically involved in the production of active forms of certain clotting factors, and certain other metabolic processes involving the binding of calcium ion.
Drugs and poisons in the class
The simplest synthetic molecule in the 4-hydroxycoumarin class is warfarin, in which the aromatic 3-position substituent is a simple phenyl group. So called "super-warfarins" or second-generation anticoagulants in this class, were developed as rodenticides for rodents that have developed warfarin resistance. The second generation agents have even larger lipid-soluble substituents at the 3-position (e.g. brodifacoum), a chemical change that causes their half life in the body to be greatly increased (sometimes to months). The rodenticide chemicals are sometimes incorrectly referred to as "coumadins" rather than 4-hydroxycoumarins (Coumadin is a brandname for warfarin). They are also referred to as "coumarins," in reference to their derivation, although this term also may be deceptive since coumarin itself, as noted, is not active in clotting, and is used mostly as a perfumery agent.
Pharmaceutical examples of 4-hydroxycoumarin pharmaceuticals include:
Compounds in this class have also been used as pesticides, specifically rodenticides. They act by causing the affected animal to hemorrhage, causing it to seek water, and thus leave dwellings to die outdoors.
The second-generation vitamin K antagonist agents, used only in this fashion as poisons (because their duration of action is too long to be used as pharmaceuticals) include:
Structures
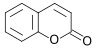


Coumarin
This molecule does not affect coagulation4-Hydroxycoumarin
This molecule does not affect coagulation, but is a known carcinogen in diesel fumes and tobacco smoke, where it probably derives from burning the additive coumarin.Dicumarol
This molecule was the first discovered 4-hydroxycoumarin anticoagulant. It is a dimer type structure connected at the 3 ring position.


Phenprocoumon
(anticoagulant)Warfarin
Most commonly used anticoagulant pharmaceuticalAcenocoumarol
(anticoagulant)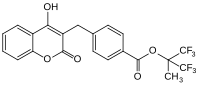
Tecarfarin (experimental anticoagulant)


Brodifacoum
This molecule is a second-generation anticoagulant with a large 3-position substituent which causes it to be retained in fatty tissues for longer times than first-generation compounds and pharmaceuticals. (rodenticide)Bromadiolone
(rodenticide)

Coumatetralyl
(rodenticide)Difenacoum
(rodenticide)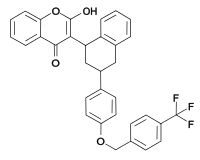
Flocoumafen
(rodenticide)
See also
External links
- 4-hydroxycoumarins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Synthesis
|
Types of coumarins
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
|
||||||||||
| glycosides | |||||||||||
| derivatives |
|
||||||||||
| Oligomers | |||||||||||
| Synthetic | |||||||||||












