
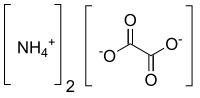
Ammonium oxalate
| |
| Names | |
|---|---|
|
IUPAC name
Diammonium ethanedioate
| |
| Other names
Diammonium oxalate
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.012.912 |
|
PubChem CID
|
|
| UNII |
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H8N2O4 | |
| Molar mass | 124.096 g·mol−1 |
| Appearance | White solid |
| Density | 1.5 g/cm3 |
| Melting point | 70 C (158 F, 343.15 K) |
| 5.20 g/100 ml (25 °C) | |
| Hazards | |
| GHS labelling: | |
| H302, H312, H319 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ammonium oxalate, C2H8N2O4 – more commonly written as (NH4)2C2O4 – is an oxalate salt with ammonium (sometimes as a monohydrate). It is a colorless (white) salt under standard conditions and is odorless and non-volatile. It is the ammonium salt of oxalic acid, and occurs in many plants and vegetables.
Vertebrate
It is produced in the body of vertebrates by metabolism of glyoxylic acid or ascorbic acid. It is not metabolized but excreted in the urine. It is a constituent of some types of kidney stone. It is also found in guano.
Mineralogy
Oxammite is a natural, mineral form of ammonium oxalate. This mineral is extremely rare.
Chemistry
Ammonium oxalate is used as an analytical reagent and general reducing agent. It and other oxalates are used as anticoagulants, to preserve blood outside the body.
Earth sciences
Acid ammonium oxalate (ammonium oxalate acidified to pH 3 with oxalic acid) is commonly employed in soil chemical analysis to extract iron and aluminium from poorly-crystalline minerals (such as ferrihydrite), iron(II)-bearing minerals (such as magnetite) and organic matter.
| Binary oxalates | |
|---|---|
| Bioxalates | |
| Oxalato complexes | |