Benfluralin
Подписчиков: 0, рейтинг: 0
| |
| Names | |
|---|---|
|
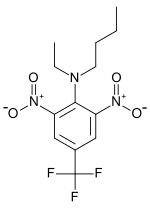
Preferred IUPAC name
N-Butyl-N-ethyl-2,6-dinitro-4-(trifluoromethyl)aniline | |
| Other names
Benefin; Benfluraline; α,α,α-Trifluoro-2,6-dinitro-N,N-ethylbutyl-p-toluidine
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.015.878 |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H16F3N3O4 | |
| Molar mass | 335.283 g·mol−1 |
| Appearance | Orange crystalline solid |
| Density | 1.338 g/mL |
| Melting point | 65.0 to 65.5 °C (149.0 to 149.9 °F; 338.1 to 338.6 K) |
| Boiling point | 121 to 122 °C (250 to 252 °F; 394 to 395 K) at 0.6 mbar |
| 1 mg/L | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benfluralin is an herbicide of the dinitroaniline class. The mechanism of action of benfluralin involves inhibition of root and shoot development.
It is used to control grasses and other weeds. Annual use in the United States was approximately 700,000 pounds (320,000 kg) in 2004.
