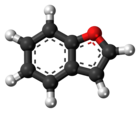
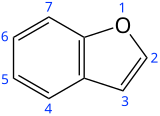
Benzofuran
Подписчиков: 0, рейтинг: 0
This article is about the heterocyclic chemical compound. For its purportedly recreational derivative drug, nicknamed "Benzo Fury", see 6-APB.
| |||
|
| |||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
1-Benzofuran | |||
| Other names
Benzofuran
Coumarone Benzo[b]furan | |||
| Identifiers | |||
|
|||
|
3D model (JSmol)
|
|||
| 107704 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider |
|
||
| DrugBank |
|
||
| ECHA InfoCard | 100.005.439 | ||
| EC Number |
|
||
| 260881 | |||
| KEGG |
|
||
|
PubChem CID
|
|||
| RTECS number |
|
||
| UNII | |||
| UN number | 1993 | ||
|
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H6O | |||
| Molar mass | 118.135 g·mol−1 | ||
| Melting point | −18 °C (0 °F; 255 K) | ||
| Boiling point | 173 °C (343 °F; 446 K) | ||
| Hazards | |||
| GHS labelling: | |||
 
|
|||
| Warning | |||
| H226, H351, H412 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P273, P280, P281, P303+P361+P353, P308+P313, P370+P378, P403+P235, P405, P501 | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
500 mg/kg (mice). | ||
| Related compounds | |||
|
Related compounds
|
Benzothiophene, Indole, Indene | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
Production
Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol.
Laboratory methods
Benzofurans can be prepared by various methods in the laboratory. Notable examples include:
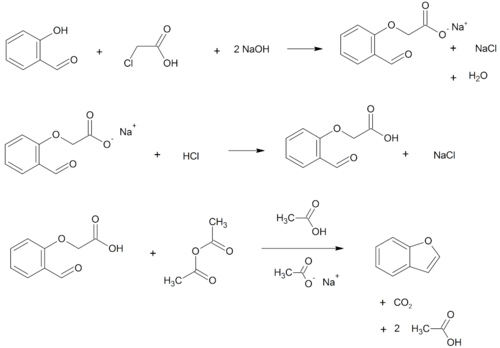
- O-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation.
- Perkin rearrangement, where a coumarin is reacted with a hydroxide:
- Diels–Alder reaction of nitro vinyl furans with various dienophiles:
Related compounds
- Substituted benzofurans
- Dibenzofuran, an analog with a second fused benzene ring.
- Furan, an analog without the fused benzene ring.
- Indole, an analog with a nitrogen instead of the oxygen atom.
- Benzothiophene, an analog with a sulfur instead of the oxygen atom.
- Isobenzofuran, the isomer with oxygen in the adjacent position.
- Aurone
- Thunberginol F
| 1 ring |
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 rings |
|
||||||||||||
| Authority control: National |
|---|