
Biosafety level
A biosafety level (BSL), or pathogen/protection level, is a set of biocontainment precautions required to isolate dangerous biological agents in an enclosed laboratory facility. The levels of containment range from the lowest biosafety level 1 (BSL-1) to the highest at level 4 (BSL-4). In the United States, the Centers for Disease Control and Prevention (CDC) have specified these levels in a publication referred to as BMBL. In the European Union, the same biosafety levels are defined in a directive. In Canada the four levels are known as Containment Levels. Facilities with these designations are also sometimes given as P1 through P4 (for pathogen or protection level), as in the term P3 laboratory.
At the lowest level of biosafety, precautions may consist of regular hand-washing and minimal protective equipment. At higher biosafety levels, precautions may include airflow systems, multiple containment rooms, sealed containers, positive pressure personnel suits, established protocols for all procedures, extensive personnel training, and high levels of security to control access to the facility. Health Canada reports that world-wide until 1999 there were recorded over 5,000 cases of accidental laboratory infections and 190 deaths.
History
The first prototype Class III (maximum containment) biosafety cabinet was fashioned in 1943 by Hubert Kaempf Jr., then a U.S. Army soldier, under the direction of Arnold G. Wedum, Director (1944–69) of Industrial Health and Safety at the United States Army Biological Warfare Laboratories, Camp Detrick, Maryland. Kaempf was tired of his MP duties at Detrick and was able to transfer to the sheet metal department working with the contractor, the H.K. Ferguson Co.
On 18 April 1955, fourteen representatives met at Camp Detrick in Frederick, Maryland. The meeting was to share knowledge and experiences regarding biosafety, chemical, radiological, and industrial safety issues that were common to the operations at the three principal biological warfare (BW) laboratories of the U.S. Army. Because of the potential implication of the work conducted at biological warfare laboratories, the conferences were restricted to top level security clearances. Beginning in 1957, these conferences were planned to include non-classified sessions as well as classified sessions to enable broader sharing of biological safety information. It was not until 1964, however, that conferences were held in a government installation not associated with a biological warfare program.
Over the next ten years, the biological safety conferences grew to include representatives from all federal agencies that sponsored or conducted research with pathogenic microorganisms. By 1966, it began to include representatives from universities, private laboratories, hospitals, and industrial complexes. Throughout the 1970s, participation in the conferences continued to expand and by 1983 discussions began regarding the creation of a formal organization. The American Biological Safety Association (ABSA) was officially established in 1984 and a constitution and bylaws were drafted the same year. As of 2008, ABSA includes some 1,600 members in its professional association.
In 1977, Jim Peacock of the Australian Academy of Science asked Bill Snowdon, then chief of the CSIRO's Australian Animal Health Laboratory (AAHL) if he could have the newly released United States' National Institutes of Health and the British equivalent requirements for the development of infrastructure for bio-containment reviewed by AAHL personnel with a view to recommending the adoption of one of them by Australian authorities. The review was carried out by CSIRO AAHL Project Manager Bill Curnow and CSIRO Engineer Arthur Jenkins. They drafted outcomes for each of the levels of security. AAHL was notionally classified as "substantially beyond P4". These were adopted by the Australian Academy of Science and became the basis for Australian legislation. It opened in 1985 costing $185 million, built on Corio Oval. The Australian Animal Health Laboratory is a Class 4/ P4 Laboratory.
In 2003, the Chinese Academy of Sciences approved the construction of mainland China's first BSL-4 laboratory at the Wuhan Institute of Virology (WIV). In 2014, the WIV's National Bio-safety Laboratory was built at a cost of 300 million yuan (US$44 million), in collaboration and with assistance from the French government's CIRI lab.
In 2007 a scientific review paper stated that the Canadian Science Centre for Human and Animal Health, which was designed in the early 1990s, "has become the prototype for modern BSL4 laboratories".
Starting with the 2020 Covid-19 pandemic near the facilities of the WIV, work in biocontainment facilities has been politicized, especially in the US Senate for example as the result of Rand Paul's work. Russia asked questions on 25 October 2022 in the United Nations over the presence in Ukraine of biolabs. In the month of April 2023 the descent into civil war of Sudan caused worries at the World Health Organization over its National Public Laboratory as contending factions battled over its area and one kicked out the NPL staff and installed a military base in its premises. At the time the facility contained organisms rated at BSL2.
Levels
Biosafety level 1
Biosafety level 1 (BSL-1) is suitable for work with well-characterized agents which do not cause disease in healthy humans. In general, these agents should pose minimal potential hazard to laboratory personnel and the environment. At this level, precautions are limited relative to other levels. Laboratory personnel must wash their hands upon entering and exiting the lab. Research with these agents may be performed on standard open laboratory benches without the use of special containment equipment. However, eating and drinking are generally prohibited in laboratory areas. Potentially infectious material must be decontaminated before disposal, either by adding a chemical such as bleach or isopropanol or by packaging for decontamination elsewhere. Personal protective equipment is only required for circumstances where personnel might be exposed to hazardous material. BSL-1 laboratories must have a door which can be closed to limit access to the lab. However, it is not necessary for BSL-1 labs to be isolated from the general building.
This level of biosafety is appropriate for work with several kinds of microorganisms including non-pathogenic strains of Escherichia coli and Staphylococcus, Bacillus subtilis, Saccharomyces cerevisiae and other organisms not suspected to contribute to human disease. Due to the relative ease and safety of maintaining a BSL-1 laboratory, these are the types of laboratories generally used as teaching spaces for high schools and colleges.
Biosafety level 2
At this level, all precautions used at Biosafety Level 1 are followed, and some additional precautions are taken. BSL-2 differs from BSL-1 in that:
- "laboratory personnel have specific training in handling pathogenic agents and are directed by competent scientists."
- Access to the laboratory is limited when work is being conducted.
- Certain procedures in which infectious aerosols or splashes may be created are conducted in biological safety cabinets or other physical containment equipment.
- Extreme precautions are taken with contaminated sharp items.
Biosafety level 2 is suitable for work involving agents of moderate potential hazard to personnel and the environment. This includes various microbes that cause mild disease to humans, or are difficult to contract via aerosol in a lab setting. Examples of pathogens classified as "Risk Group 2" in the United States include hepatitis A, B, and C viruses, human immunodeficiency virus (HIV), pathogenic strains of Escherichia coli and Staphylococcus, Salmonella, Plasmodium falciparum, and Toxoplasma gondii. Notably, the European Union departs from the United States and classifies HIV and hepatitis B – G as Risk Group 3 agents best handled at BSL-3.
Prions, the infectious agents that transmit prion diseases such as vCJD, are typically handled under Biosafety Level 2 or higher. This is due to the lack of any evidence of aerosol transmission and relatively higher infective dose of prion diseases, though some circumstances (such as handling animal-infective prions in a facility which cares for vulnerable animals) would require BSL-3 conditions.
Biosafety level 3

Biosafety level 3 is appropriate for work involving microbes which can cause serious and potentially lethal disease via the inhalation route. This type of work can be done in clinical, diagnostic, teaching, research, or production facilities. Here, the precautions undertaken in BSL-1 and BSL-2 labs are followed, as well as additional measures including:
- A laboratory-specific biosafety manual must be drafted which details how the laboratory will operate in compliance with all safety requirements.
- All laboratory personnel are provided medical surveillance and offered relevant immunizations (where available) to reduce the risk of an accidental or unnoticed infection.
- All procedures involving infectious material must be done within a biological safety cabinet.
- Laboratory personnel must wear solid-front protective clothing (i.e. gowns that tie in the back). This cannot be worn outside of the laboratory and must be discarded or decontaminated after each use.
In addition, the facility which houses the BSL-3 laboratory must have certain features to ensure appropriate containment. The entrance to the laboratory must be separated from areas of the building with unrestricted traffic flow. Additionally, the laboratory must be behind two sets of self-closing doors (to reduce the risk of aerosols escaping). The construction of the laboratory is such that it can be easily cleaned. Carpets are not permitted, and any seams in the floors, walls, and ceilings are sealed to allow for easy cleaning and decontamination. Additionally, windows must be sealed, and a ventilation system installed which forces air to flow from the "clean" areas of the lab to the areas where infectious agents are handled. Air from the laboratory must be filtered before it can be recirculated.
A 2015 study by USA Today journalists identified more than 200 lab sites in the U.S. that were accredited biosafety levels 3 or 4. The Proceedings of a Workshop on "Developing Norms for the Provision of Biological Laboratories in Low-Resource Contexts" provides a list of BSL-3 laboratories in those countries.
Biosafety level 3 is commonly used for research and diagnostic work involving various microbes which can be transmitted by aerosols and/or cause severe disease. These include Francisella tularensis, Mycobacterium tuberculosis, Chlamydia psittaci, Venezuelan equine encephalitis virus, Eastern equine encephalitis virus, SARS-CoV-1, MERS-CoV, Coxiella burnetii, Rift Valley fever virus, Rickettsia rickettsii, several species of Brucella, chikungunya, yellow fever virus, West Nile virus, Yersinia pestis, and SARS-CoV-2.
Biosafety level 4
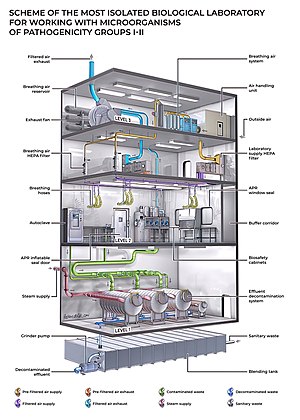
Biosafety level 4 (BSL-4) is the highest level of biosafety precautions, and is appropriate for work with agents that could easily be aerosol-transmitted within the laboratory and cause severe to fatal disease in humans for which there are no available vaccines or treatments. BSL-4 laboratories are generally set up to be either cabinet laboratories or protective-suit laboratories. In cabinet laboratories, all work must be done within a class III biosafety cabinet. Materials leaving the cabinet must be decontaminated by passing through an autoclave or a tank of disinfectant. The cabinets themselves are required to have seamless edges to allow for easy cleaning. Additionally, the cabinet and all materials within must be free of sharp edges to reduce the risk of damage to the gloves. In a protective-suit laboratory, all work must be done in a class II biosafety cabinet by personnel wearing a positive pressure suit. To exit the BSL-4 laboratory, personnel must pass through a chemical shower for decontamination, then a room for removing the positive-pressure suit, followed by a personal shower. Entry into the BSL-4 laboratory is restricted to trained and authorized individuals, and all persons entering and exiting the laboratory must be recorded.
As with BSL-3 laboratories, BSL-4 laboratories must be separated from areas that receive unrestricted traffic. Additionally, airflow is tightly controlled to ensure that air always flows from "clean" areas of the lab to areas where work with infectious agents is being performed. The entrance to the BSL-4 lab must also employ airlocks to minimize the possibility that aerosols from the lab could be removed from the lab. All laboratory waste, including filtered air, water, and trash must also be decontaminated before it can leave the facility.
Biosafety level 4 laboratories are used for diagnostic work and research on easily transmitted pathogens which can cause fatal disease. These include a number of viruses known to cause viral hemorrhagic fever such as Marburg virus, Ebola virus, Lassa virus, and Crimean-Congo hemorrhagic fever. Other pathogens handled at BSL-4 include Hendra virus, Nipah virus, and some flaviviruses. Additionally, poorly characterized pathogens which appear closely related to dangerous pathogens are often handled at this level until sufficient data are obtained either to confirm continued work at this level, or to permit working with them at a lower level. This level is also used for work with Variola virus, the causative agent of smallpox, though this work is only performed at the Centers for Disease Control and Prevention in Atlanta, United States, and the State Research Center of Virology and Biotechnology in Koltsovo, Russia.
The circular containment tube separates the patient table in the "hot" zone (pathogen present) from the "cold" zone around this MRI machine
Inside a Class III biological safety cabinet with an aerosol control platform
Effluent decontamination system of a BSL-4 lab of NIAID
BSL-4 facilities for extraterrestrial samples
Sample-return missions that bring back to Earth samples obtained from a Category V body must be curated at facilities rated BSL-4. Because the existing BSL-4 facilities in the world do not provide the level of cleanliness necessary to such pristine samples, there is a need to design a facility dedicated to curation of restricted (potentially biohazardous) extraterrestrial materials. The systems of such facilities must be able to contain unknown biohazards, as the sizes of any putative alien microorganisms are unknown. Ideally, it should filter particles down to 10 nanometers, and release of a particle 50 nanometers or larger is unacceptable under any circumstance.
Because NASA and ESA are collaborating on the Mars Sample Return campaign, due to return samples from Mars in the early 2030s, the need for a Sample Receiving Facility (SRF) is becoming more pressing. An SRF is expected to take 7 to 10 years from design to completion, and an additional two years is recommended for the staff to become proficient and accustomed to the facilities.
List of BSL-4 facilities
According to a U.S. Government Accountability Office (GAO) report published on 4 October 2007, a total of 1,356 CDC/USDA registered BSL-3 facilities were identified throughout the United States. Approximately 36% of these laboratories are located in academia. 15 BSL-4 facilities were identified in the U.S. in 2007, including nine at federal labs. As of May 2021, there are 42 BSL-4 facilities in operation around the world, with a further 17 planned or under construction.
The following is a list of existing BSL-4 facilities worldwide.
| Country | Location | Name | Date established |
Description |
|---|---|---|---|---|
| Argentina | Buenos Aires | National Service of Healthcare and Agriculture Quality (SENASA) | Diagnostic laboratory for foot-and-mouth disease. | |
| Australia | Geelong, Victoria | Australian Centre for Disease Preparedness | 1985 | Capable of housing from large experimental animals to insects under conditions that exceed all BSL 4 requirements. The antecedent of all such facilities developed since the 1980s. Arguably the most researched design and construction project ever. The ACDP is subdivided into a number of isolation zones that can be managed at differing containment levels concurrently. CSIRO AAHL Project Manager and Architect, William Curnow, provided technical reviews to Canadian, Indian, UK and French Authorities and consulted with Dr Jerry Callis [PIADC] to UN FAO on matters of bio-containment. Formerly known as the Australian Animal Health Laboratory (AAHL) and renamed to Australian Centre for Disease Preparedness April 2020 |
| Melbourne | University of Melbourne – Doherty Institute for Infection and Immunity | 2014 | Diagnostic reference lab. | |
| National High Security Laboratory | Operates under the auspice of the Victoria Infectious Diseases Reference Laboratory. | |||
| Belarus | Minsk | Republican Research and Practical Center for Epidemiology and Microbiology (RPPCM) | Formerly the SRIEM. | |
| Brazil | Pedro Leopoldo, Minas Gerais | Laboratório Nacional Agropecuário de Minas Gerais (Lanagro/MG) | 2014 | Focus on Agropecuary diseases and diagnostics, like the foot-and-mouth disease. |
| Campinas, São Paulo | Laboratório Nacional de Máxima Contenção Biológica (LNMCB) | 2022–2023? (expected) | It was announced in 2021 to be built near the Sincrotron lab. | |
| Canada | Winnipeg, Manitoba | National Microbiology Laboratory | 1999 | Located at the Canadian Science Centre for Human and Animal Health, it is jointly operated by the Public Health Agency of Canada and the Canadian Food Inspection Agency. |
| China | Wuhan, Hubei | Wuhan Institute of Virology of the Chinese Academy of Sciences | 2015 | Wuhan Institute of Virology has existed since 1956 and already hosted BSL-3 laboratories. A BSL-4 facility was completed in 2015, and became the first BSL-4 laboratory in China. |
| Harbin, Heilongjiang | Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences | 2018 | Harbin Veterinary Research Institute researches prevention and control of major infectious diseases. China's second, and the first for large animals, BSL-4 lab. | |
| Czech Republic | Těchonín, Pardubice Region | Biological Defense Center | 1971, rebuilt 2003–2007 | Hospital and research facility. Located at the Centrum biologické ochrany (Biological Defense Center). Operated by Army of the Czech Republic. |
| France | Brétigny-sur-Orge, Essonne | French Armed Biomedical Research Institute, French Defence Health Service | French Army laboratory. | |
| Lyon, Metropolis of Lyon | Jean Mérieux BSL-4 Laboratory | 1999 | Built and owned by the Fondation Mérieux. Since 2004, operated by INSERM. | |
| Vert-le-Petit, Essonne | Laboratoire de la DGA | 2013 | Operated by the Ministry of Defense. | |
| Gabon | Franceville, Haut-Ogooué Province | Centre International de Recherches Médicales de Franceville | This facility is operated by a research organization supported by both Gabonese (mainly) and French governments, and is West Africa's only P4 lab (BSL-4). | |
| Germany | Berlin | Robert Koch Institute | 2015 | Diagnostic and experimental lab facility. |
| Hamburg | Bernhard Nocht Institute for Tropical Medicine | 2014 | Part of the Leibniz Center Infection. National reference lab for tropical viruses. | |
| Isle of Riems, Greifswald, Mecklenburg-Vorpommern | Friedrich Loeffler Institute | 2010 | Focus on animal viral diseases and diagnostics. | |
| Marburg, Hesse | Philipps University of Marburg | 2008 | Focuses on hemorrhagic fever viruses. | |
| Hungary | Budapest | National Center for Epidemiology | 1998 | Division of Virology operates three WHO National Reference Laboratories. The BSL-4 biosafety laboratory provides a modern means to process dangerous imported zoonotic viral pathogens. |
| Pécs | University of Pécs | 2016 | Opened in 2016, part of Szentágothai János Kutatóközpont. | |
| India | Bhopal, Madhya Pradesh | National Institute of High Security Animal Disease | 1998 | This facility deals especially to zoonotic organisms and emerging infectious disease threats. |
| Hyderabad, Telangana | Centre for Cellular and Molecular Biology | 2009 | National BSL-4 Containment Facility for Human Infectious Diseases. | |
| Pune, Maharashtra | National Institute of Virology | 2012 | India's most advanced BSL-4 category lab. | |
| Italy | Rome, Lazio | Istituto Nazionale per le Malattie Infettive | 1997 | The "National Institute of Infectious Diseases" used to operate within the Lazzaro Spallanzani hospital; the facility is now independent and is home to five BSL-3 labs as well as a single BSL-4 laboratory, which was completed in 1997. |
| Milan, Lombardy | Ospedale Luigi Sacco | 2006 | ||
| Japan | Musashimurayama, Tokyo | National Institute for Infectious Diseases | 2015 | Located at National Institute for Infectious Diseases, Department of Virology I. Built in 1981; operated at BSL-3 until 2015 due to opposition from nearby residents. |
| Tsukuba, Ibaraki Prefecture | Institute of Physical and Chemical Research (RIKEN) | 1984 | Facility completed in 1984 but not operated as BSL-4 due to local opposition. | |
| Philippines | New Clark City, Capas, Tarlac | Virology Institute of the Philippines | 2024 (expected) | First BSL-4 Lab in the Philippines when completed. |
| Russia | Sergiyev Posad, Moscow Oblast | 48th Central Scientific Research Institute Sergiev Posad | ||
| Koltsovo, Novosibirsk Oblast | State Research Center of Virology and Biotechnology (VECTOR) | One of two WHO-approved facilities for work on variola virus (AKA smallpox). | ||
| Singapore | Singapore | DSO National Laboratories | End-2025 (expected) | First BSL-4 Lab in Singapore when completed. |
| South Africa | Johannesburg, Gauteng | National Institute for Communicable Diseases | 2002 | |
| South Korea | Cheongju, North Chungcheong Province | Korea Centers for Disease Control and Prevention | 2017 | First BSL-4 Lab in South Korea. |
| Sweden | Solna, Stockholm County | Public Health Agency of Sweden | 2001 | The only BSL-4 facility in the Nordic region. Constructed for research and diagnostics of hemorrhagic fever viruses. |
| Switzerland | Geneva, Canton of Geneva | University Hospital of Geneva | "Glove box" type laboratory; primarily for handling clinical samples. | |
| Spiez, Canton of Bern | Spiez Laboratory | 2013 | Run by the Federal Office for Civil Protection of the Federal Department of Defence, Civil Protection and Sports. | |
| Mittelhäusern, Canton of Bern | The Institute of Virology and Immunology IVI | Part of the Food Safety and Veterinary Office (FSVO). Primary purpose is diagnostics of highly pathogenic viruses. | ||
| Republic of China (Taiwan) | National Defense University | Institute of Preventive Medicine | 1983 | |
| Taipei, Taiwan | Kwen-yang Laboratory | [1] | ||
| United Kingdom | Camden, Greater London | Francis Crick Institute | 2015 | Has BSL-4 space but does not work on human pathogens. |
| Colindale, Greater London | Public Health England's Centre for Infections | Department of Health laboratory. Diagnostics for various viral diseases. Part of the European Network of Biosafety-Level-4 Laboratories. | ||
| Mill Hill, Greater London | National Institute for Medical Research | Medical Research Council laboratory. Research and diagnostics for highly pathogenic viruses. Closed in 2017 and work moved to the Francis Crick Institute. Site demolished in 2018. | ||
| Potters Bar, Hertfordshire | National Institute for Biological Standards and Control | Department of Health and Home Office laboratory. Develop assays and reagents for research on virulent pathogens. | ||
| Addlestone, Surrey | Animal and Plant Health Agency | Department for Environment, Food and Rural Affairs laboratory. Diagnostics and research for animal diseases. | ||
| Pirbright, Surrey | Institute for Animal Health | Biotechnology and Biological Sciences Research Council laboratory. Research on highly pathogenic animal diseases. | ||
| Merial Animal Health | Private lab. Produces vaccines against foot and mouth disease and bluetongue disease. | |||
| Porton Down, Wiltshire | Centre for Emergency Preparedness and Response | Department of Health laboratory. Diagnostics and research for haemorrhagic fever viruses. Part of the European Network of Biosafety-Level-4 Laboratories. | ||
| Defence Science and Technology Laboratory | Ministry of Defence laboratory. Focuses on protection from biological weapons. | |||
| United States | Fort Collins, Colorado | Centers for Disease Control and Prevention, Division of Vector Borne Diseases | A BSL 3/4 facility that operates in connection with some of Colorado State University's biomedical research programs. The location specializes in arboviral and bacterial diseases. | |
| Atlanta, Georgia | Centers for Disease Control and Prevention | Currently operates in two buildings. One of two facilities in the world that officially hold smallpox. | ||
| Georgia State University | 1997 | Research focus on B virus. | ||
| Manhattan, Kansas | National Bio and Agro-Defense Facility (NBAF), Kansas State University | 2022 (expected) | Under construction. Facility to be operated by the Department of Homeland Security, and replace the Plum Island Animal Disease Center. Expected to be operational by 2022–2023. | |
| Bethesda, Maryland | National Institutes of Health (NIH) | Located on the NIH Campus, it currently only operates with BSL-3 agents. | ||
| Fort Detrick, Maryland | Integrated Research Facility | Operated by National Institute of Allergy and Infectious Diseases (NIAID). Focuses on animal models of human diseases. | ||
| National Biodefense Analysis and Countermeasures Center | Operated by the Department of Homeland Security. Focus on potential bioterrorism threats. | |||
| US Army Medical Research Institute of Infectious Diseases (USAMRIID) | 1969 | Run by the U.S. Army. Research focuses on biological threats to the U.S. military. | ||
| Boston, Massachusetts | National Emerging Infectious Diseases Laboratory (NEIDL), Boston University | Built 2008, Opened 2012, BSL-4 Approval in 2017 | Focus on potential threats to public health. Operated by Boston University School of Medicine. | |
| Hamilton, Montana | Rocky Mountain Laboratories Integrated Research Facility | 2008 | NIAID laboratory. Focus on vector-borne diseases. | |
| Galveston, Texas | Galveston National Laboratory, National Biocontainment Facility | Opened in 2008, facility is operated by the University of Texas Medical Branch (UTMB). | ||
| Shope Laboratory | 2004 | Operated by UTMB. | ||
| San Antonio, Texas | Texas Biomedical Research Institute | 1999 | The only privately owned BSL-4 lab in the US. | |
| Richmond, Virginia | Virginia Division of Consolidated Laboratories | 2003 | A BSL-4 lab that also acts as a BSL-3 lab. |
Safety concerns
A North Carolina Mosquito & Vector Control Association (NCMVCA) study highlighted safety concerns. In the United States, laboratories can be funded by federal, state, private, non-profit, or academically. The last accounts for 72% of the funding.
High-containment labs that are registered with the Centers for Disease Control and Prevention (CDC) and the U.S. Department of Agriculture's (USDA) Select Agent Program must adhere to Department of Defense standards. Since BSL3 and 4 laboratories in the United States are regulated by either the CDC or USDA or another federal agency (depending on the pathogens they handle), no single federal agency is responsible for regulating or tracking the number of these labs. U.S. high-containment laboratories that handle pathogens which are declared as "select agents" must be inspected periodically by the CDC or USDA, adhere to certain standards, and maintain ongoing education on biosecurity and biosafety policies as mandated by law.
See also
- Aeromedical Isolation Team
- Biocontainment
- Biological hazard
- Biosafety
- Hazmat suit
- Interplanetary contamination
- Laboratory Response Network
- List of laboratory biosecurity incidents
- Safety engineering
- Security engineering
- Select agent
External links
- Biosafety in Microbiological and Biomedical Laboratories, CDC publication
- Federation of American Scientists: Biosafety Level 3 and 4 Labs

![Regular inspection of positive-pressure suits to locate any leaks[33]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/62/NIAID_Integrated_Research_Facility_-_Positive_Pressure_Personnel_Suit_Inspection.jpg/200px-NIAID_Integrated_Research_Facility_-_Positive_Pressure_Personnel_Suit_Inspection.jpg)





