
Denintuzumab mafodotin
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | CD19 |
| Clinical data | |
| Other names | SGN-19A |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| KEGG | |
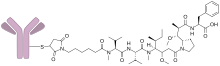
Denintuzumab mafodotin (INN; development codes SGN-19A or SGN-CD19A) is a humanized monoclonal antibody-drug conjugate designed for the treatment of CD19-positive acute lymphoblastic leukemia and B-cell non-Hodgkin lymphoma. It consists of an anti-CD19 mAb linked to monomethyl auristatin F (MMAF), a cytotoxic agent. This drug was developed by Seattle Genetics.
Denintuzumab refers to the anti-CD19 antibody, and mafodotin refers to MMAF and the chemical linkage.
Clinical trials
The drug is in phase I clinical trials. Preliminary phase I results for B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL) and B-lineage acute lymphocytic leukemia (B-ALL) were presented at the ASH medical conference Dec 2015.
Phase 2
A separate randomized phase 2 trial started in 2015 to evaluate SGN-CD19A in combination with R-ICE chemotherapy for second-line DLBCL. A phase 2 clinical trial in front-line DLBCL is started in 2016. Both trials were terminated by the sponsor based on portfolio prioritization.