
FV-100
Подписчиков: 0, рейтинг: 0
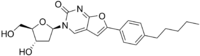
| |
| Names | |
|---|---|
|
IUPAC name
3-(2-Deoxy-β-D-erythro-pentofuranosyl)-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one
| |
|
Systematic IUPAC name
3-[(2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)oxolan-2-yl]-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one | |
| Other names
Cf1743
| |
| Identifiers | |
|
3D model (JSmol)
|
|
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C22H26N2O5 | |
| Molar mass | 398.459 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
FV-100, also known as Cf1743, is an orally available nucleoside analogue drug with antiviral activity. It may be effective against shingles.
It was discovered in 1999 in the laboratories of Prof Chris McGuigan, Welsh School of Pharmacy and Prof. Jan Balzarini, Rega Institute, Leuven, Belgium.
Clinical trials
FV-100 was tested against valaciclovir in a phase II trial in patients with herpes zoster. The trial was sponsored by Bristol-Myers Squibb. The drug is currently being developed by ContraVir Pharmaceuticals, Inc., Edison, New Jersey. It has reached Phase III clinical trials.
| Baltimore I |
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis B (VII) | |||||||||||||||||||||
| Multiple/general |
|
||||||||||||||||||||
| |||||||||||||||||||||