
Fenbufen
Подписчиков: 0, рейтинг: 0
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.148 |
| Chemical and physical data | |
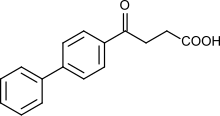
| Formula | C16H14O3 |
| Molar mass | 254.285 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 186 °C (367 °F) |
| |
| |
|
| |
Fenbufen is a nonsteroidal anti-inflammatory drug used to treat pain.
Fenbufen is a member of the propionic acid derivatives class of drugs.
It was introduced by American Cyanamid under the trade name Lederfen in the 1980s. Due to liver toxicity, it was withdrawn from markets in the developed world in 2010.
As of 2015 it was available in Taiwan and Thailand under several brand names.
Preparation
Fenbufen can be synthesized by acylation of biphenyl with succinic anhydride under Friedel-Crafts conditions.
|
pyrazolones / pyrazolidines |
|
|---|---|
| salicylates | |
|
acetic acid derivatives and related substances |
|
| oxicams | |
|
propionic acid derivatives (profens) |
|
|
n-arylanthranilic acids (fenamates) |
|
|
COX-2 inhibitors (coxibs) |
|
| other | |
| NSAID combinations |
|
Key: underline indicates initially developed first-in-class compound of specific group; #WHO-Essential Medicines; †withdrawn drugs; ‡veterinary use. | |