
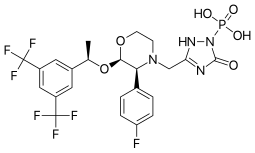
Fosaprepitant
Подписчиков: 0, рейтинг: 0
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Emend, Ivemend |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a604003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | >95% (aprepitant) |
| Metabolism | To aprepitant |
| Elimination half-life | 9 to 13 hours (aprepitant) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H22F7N4O6P |
| Molar mass | 614.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Fosaprepitant (Emend for Injection (US), Ivemend (EU)) is an antiemetic medication, administered intravenously. It is a prodrug of aprepitant.
Fosaprepitant was developed by Merck & Co. and was approved for medical use in the United States, and in the European Union in January 2008.