
Indium phosphide

| |
| |
| Names | |
|---|---|
| Other names
Indium(III) phosphide
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider |
|
| ECHA InfoCard | 100.040.856 |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| InP | |
| Molar mass | 145.792 g/mol |
| Appearance | black cubic crystals |
| Density | 4.81 g/cm3, solid |
| Melting point | 1,062 °C (1,944 °F; 1,335 K) |
| Solubility | slightly soluble in acids |
| Band gap | 1.344 eV (300 K; direct) |
| Electron mobility | 5400 cm2/(V·s) (300 K) |
| Thermal conductivity | 0.68 W/(cm·K) (300 K) |
|
Refractive index (nD)
|
3.1 (infrared); 3.55 (632.8 nm) |
| Structure | |
| Zinc blende | |
|
a = 5.8687 Å
|
|
| Tetrahedral | |
| Thermochemistry | |
|
Heat capacity (C)
|
45.4 J/(mol·K) |
|
Std molar
entropy (S⦵298) |
59.8 J/(mol·K) |
|
Std enthalpy of
formation (ΔfH⦵298) |
-88.7 kJ/mol |
|
Gibbs free energy (ΔfG⦵)
|
-77.0 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
|
Main hazards
|
Toxic, hydrolysis to phosphine |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
|
Other anions
|
Indium nitride Indium arsenide Indium antimonide |
|
Other cations
|
Aluminium phosphide Gallium phosphide |
|
Related compounds
|
Indium gallium phosphide Aluminium gallium indium phosphide Gallium indium arsenide antimonide phosphide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
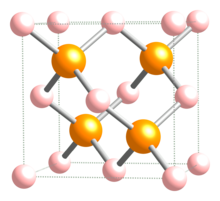
Indium phosphide (InP) is a binary semiconductor composed of indium and phosphorus. It has a face-centered cubic ("zincblende") crystal structure, identical to that of GaAs and most of the III-V semiconductors.
Manufacturing
Indium phosphide can be prepared from the reaction of white phosphorus and indium iodide at 400 °C., also by direct combination of the purified elements at high temperature and pressure, or by thermal decomposition of a mixture of a trialkyl indium compound and phosphine.
Applications
The application fields of InP splits up into three main areas. It is used as the basis for optoelectronic components, high-speed electronics, and photovoltaics
High-speed optoelectronics
InP is used as a substrate for epitaxial optoelectronic devices based other semiconductors, such as indium gallium arsenide. The devices include pseudomorphic heterojunction bipolar transistors that could operate at 604 GHz.
InP itself has a direct bandgap, making it useful for optoelectronics devices like laser diodes and photonic integrated circuits for the optical telecommunications industry, to enable wavelength-division multiplexing applications. It is used in high-power and high-frequency electronics because of its superior electron velocity with respect to the more common semiconductors silicon and gallium arsenide. It is used in lasers, sensitive photodetectors and modulators in the wavelength window typically used for telecommunications, i.e., 1550 nm wavelengths, as it is a direct bandgap III-V compound semiconductor material. The wavelength between about 1510 nm and 1600 nm has the lowest attenuation available on optical fibre (about 0.2 dB/km).
Photovoltaics and optical sensing
InP can be used in photonic integrated circuits that can generate, amplify, control and detect laser light.
Optical sensing applications of InP include
- Air pollution control by real-time detection of gases (CO, CO2, NOX [or NO + NO2], etc.).
- Quick verification of traces of toxic substances in gases and liquids, including tap water, or surface contaminations.
- Spectroscopy for non-destructive control of product, such as food. Researchers of Eindhoven University of Technology and MantiSpectra have already demonstrated the application of an integrated near-infrared spectral sensor for milk. In addition, it has been proven that this technology can also be applied to plastics and illicit drugs.
Cited sources
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
External links
- Extensive site on the physical properties of indium phosphide (Ioffe institute)
- InP conference series at IEEE
- Indium Phosphide: Transcending frequency and integration limits. Semiconductor TODAY Compounds&AdvancedSilicon • Vol. 1 • Issue 3 • September 2006
|
Indium compounds
| |||
|---|---|---|---|
| Indium(I) |
|
||
| Indium(I,III) | |||
| Indium(III) |
|
||
|
Phosphorus compounds
| |
|---|---|
| Binary phosphides |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ternary phosphides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quaternary phosphides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quinary phosphides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| See also | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
