
Liafensine
Подписчиков: 0, рейтинг: 0
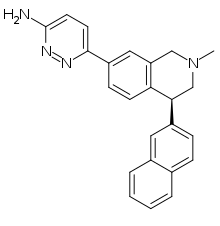 | |
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H22N4 |
| Molar mass | 366.468 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Liafensine (BMS-820836) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) which was under development by Bristol-Myers Squibb for the treatment of major depressive disorder. Though it demonstrated comparable effectiveness to escitalopram and duloxetine in phase II clinical trials, development was paused in 2013 because liafensine failed to show superior effectiveness relative to these drugs, a decision that was made likely based on its increased capacity for side effects as well as potential for abuse.