Lisdexamfetamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Vyvanse, Tyvense, Elvanse, others |
| Other names |
L-Lysine-d-amphetamine; (2S)-2,6-Diamino-N-[(2S)-1-phenylpropan-2-yl]hexanamide N-[(2S)-1-Phenyl-2-propanyl]-L-lysinamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607047 |
| License data |
|
| Pregnancy category |
|
| Dependence liability |
Moderate |
| Addiction liability |
Moderate |
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 96.4% |
| Protein binding | 20% (as active amphetamine) |
| Metabolism | Hydrolysis by enzymes in red blood cells initially, subsequent metabolism follows |
| Metabolites | Dextroamphetamine (and its metabolites) and L-lysine |
| Onset of action | Oral: <2 hours |
| Elimination half-life | Lisdexamfetamine: <1 hour Dextroamphetamine: 10–12 h |
| Duration of action | 10–12 hours |
| Excretion | Kidney: ~2% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H25N3O |
| Molar mass | 263.385 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Lisdexamfetamine, sold under the brand name Vyvanse among others, is a stimulant medication that is mainly used to treat attention deficit hyperactivity disorder (ADHD) in people over the age of five as well as moderate-to-severe binge eating disorder in adults. Lisdexamfetamine is taken by mouth. Its effects generally begin within two hours and last for up to 14 hours. In the United Kingdom, it is usually less preferred than methylphenidate for the treatment of children.
Common side effects of lisdexamfetamine include loss of appetite, anxiety, diarrhea, trouble sleeping, irritability, and nausea. Rare but serious side effects include mania, sudden cardiac death in those with underlying heart problems, and psychosis. It has a high potential for substance abuse per the Drug Enforcement Administration (DEA).Serotonin syndrome may occur if used with certain other medications. Its use during pregnancy may result in harm to the baby and use during breastfeeding is not recommended by the manufacturer.
Lisdexamfetamine is an inactive prodrug that works after being converted by the body into dextroamphetamine, a central nervous system (CNS) stimulant. Chemically, lisdexamfetamine is composed of the amino acid L-lysine, attached to dextroamphetamine.
Lisdexamfetamine was approved for medical use in the United States in 2007. In 2020, it was the 85th most commonly prescribed medication in the United States, with more than 8 million prescriptions. It is a Class B controlled substance in the United Kingdom and a Schedule II controlled substance in the United States.
Uses
Medical
Lisdexamfetamine is used primarily as a treatment for attention deficit hyperactivity disorder (ADHD) and binge eating disorder; it has similar off-label uses as those of other pharmaceutical amphetamines. Individuals over the age of 65 were not commonly tested in clinical trials of lisdexamfetamine for ADHD. Long-term amphetamine exposure at sufficiently high doses in some animal species is known to produce abnormal dopamine system development or nerve damage, but in some cases with humans with ADHD, pharmaceutical amphetamines at therapeutic dosages may improve brain development and nerve growth. Reviews of magnetic resonance imaging (MRI) studies suggest that long-term treatment with amphetamine decreases abnormalities in brain structure and function found in subjects with ADHD, and improves function in several parts of the brain, such as the right caudate nucleus of the basal ganglia.
Reviews of clinical stimulant research have established the safety and effectiveness of long-term continuous amphetamine use for the treatment of ADHD.Randomized controlled trials of continuous stimulant therapy for the treatment of ADHD spanning 2 years have demonstrated treatment effectiveness and safety. Two reviews have indicated that long-term continuous stimulant therapy for ADHD is effective for reducing the core symptoms of ADHD (i.e., hyperactivity, inattention, and impulsivity), enhancing quality of life and academic achievement, and producing improvements in a large number of functional outcomes across 9 categories of outcomes related to academics, antisocial behavior, driving, non-medicinal drug use, obesity, occupation, self-esteem, service use (i.e., academic, occupational, health, financial, and legal services), and social function. One review highlighted a nine-month randomized controlled trial of amphetamine treatment for ADHD in children that found an average increase of 4.5 IQ points, continued increases in attention, and continued decreases in disruptive behaviors and hyperactivity. Another review indicated that, based upon the longest follow-up studies conducted to date, lifetime stimulant therapy that begins during childhood is continuously effective for controlling ADHD symptoms and reduces the risk of developing a substance use disorder as an adult.
Current models of ADHD suggest that it is associated with functional impairments in some of the brain's neurotransmitter systems; these functional impairments involve impaired dopamine neurotransmission in the mesocorticolimbic projection and norepinephrine neurotransmission in the noradrenergic projections from the locus coeruleus to the prefrontal cortex. Psychostimulants like methylphenidate and amphetamine are effective in treating ADHD because they increase neurotransmitter activity in these systems. Approximately 80% of those who use these stimulants see improvements in ADHD symptoms. Children with ADHD who use stimulant medications generally have better relationships with peers and family members, perform better in school, are less distractible and impulsive, and have longer attention spans. The Cochrane reviews on the treatment of ADHD in children, adolescents, and adults with pharmaceutical amphetamines stated that short-term studies have demonstrated that these drugs decrease the severity of symptoms, but they have higher discontinuation rates than non-stimulant medications due to their adverse side effects. A Cochrane review on the treatment of ADHD in children with tic disorders such as Tourette syndrome indicated that stimulants in general do not make tics worse, but high doses of dextroamphetamine could exacerbate tics in some individuals.
Enhancing performance
Cognitive performance
In 2015, a systematic review and a meta-analysis of high quality clinical trials found that, when used at low (therapeutic) doses, amphetamine produces modest yet unambiguous improvements in cognition, including working memory, long-term episodic memory, inhibitory control, and some aspects of attention, in normal healthy adults; these cognition-enhancing effects of amphetamine are known to be partially mediated through the indirect activation of both dopamine receptor D1 and adrenoceptor α2 in the prefrontal cortex. A systematic review from 2014 found that low doses of amphetamine also improve memory consolidation, in turn leading to improved recall of information. Therapeutic doses of amphetamine also enhance cortical network efficiency, an effect which mediates improvements in working memory in all individuals. Amphetamine and other ADHD stimulants also improve task saliency (motivation to perform a task) and increase arousal (wakefulness), in turn promoting goal-directed behavior. Stimulants such as amphetamine can improve performance on difficult and boring tasks and are used by some students as a study and test-taking aid. Based upon studies of self-reported illicit stimulant use, 5–35% of college students use diverted ADHD stimulants, which are primarily used for enhancement of academic performance rather than as recreational drugs. However, high amphetamine doses that are above the therapeutic range can interfere with working memory and other aspects of cognitive control.
Physical performance
Amphetamine is used by some athletes for its psychological and athletic performance-enhancing effects, such as increased endurance and alertness; however, non-medical amphetamine use is prohibited at sporting events that are regulated by collegiate, national, and international anti-doping agencies. In healthy people at oral therapeutic doses, amphetamine has been shown to increase muscle strength, acceleration, athletic performance in anaerobic conditions, and endurance (i.e., it delays the onset of fatigue), while improving reaction time. Amphetamine improves endurance and reaction time primarily through reuptake inhibition and release of dopamine in the central nervous system. Amphetamine and other dopaminergic drugs also increase power output at fixed levels of perceived exertion by overriding a "safety switch", allowing the core temperature limit to increase in order to access a reserve capacity that is normally off-limits. At therapeutic doses, the adverse effects of amphetamine do not impede athletic performance; however, at much higher doses, amphetamine can induce effects that severely impair performance, such as rapid muscle breakdown and elevated body temperature.
Available forms
Lisdexamfetamine is available as the dimesylate salt in the form of both oral capsules and chewable tablets. The capsules are available in doses of 10, 20, 30, 40, 50, 60, and 70 mg, while the chewable tablets are available in doses of 10, 20, 30, 40, 50, and 60 mg. These amounts of lisdexamfetamine dimesylate are equivalent to 5.8, 11.6, 17.3, 23.1, 28.9, 34.7, and 40.5 mg lisdexamfetamine free-base, respectively. A dose of 50 mg of lisdexamfetamine dimesylate is approximately equimolar to a 20 mg dose of dextroamphetamine sulfate or to 15 mg dextroamphetamine free-base in terms of the amount of dextroamphetamine contained. Lisdexamfetamine capsules can be swallowed intact, or they can be opened and mixed into water, yogurt, or applesauce and consumed in that manner.
Contraindications
Pharmaceutical lisdexamfetamine is contraindicated in patients with hypersensitivity to amphetamine products or any of the formulation's inactive ingredients. It is also contraindicated in patients who have used a monoamine oxidase inhibitor (MAOI) within the last 14 days. Amphetamine products are contraindicated by the United States Food and Drug Administration (USFDA) in people with a history of drug abuse, heart disease, or severe agitation or anxiety, or in those currently experiencing arteriosclerosis, glaucoma, hyperthyroidism, or severe hypertension. However, a European consensus statement on adult ADHD noted that stimulants do not worsen substance misuse in adults with ADHD and comorbid substance use disorder and should not be avoided in these individuals. In any case, the statement noted that immediate-release stimulants should be avoided in those with both ADHD and substance use disorder and that slower-release stimulant formulations like OROS methylphenidate (Concerta) and lisdexamfetamine should be preferred due to their lower misuse potential. Prescribing information approved by the Australian Therapeutic Goods Administration further contraindicates anorexia.
Adverse effects
Products containing lisdexamfetamine have a comparable drug safety profile to those containing amphetamine. The major side effects of lisdexamfetamine in short-term clinical trials (≥5% incidence) have included decreased appetite, insomnia, dry mouth, weight loss, irritability, upper abdominal pain, nausea, vomiting, diarrhea, constipation, increased heart rate, anxiety, dizziness, and feeling jittery. Rates of side effects may vary in adults, adolescents, and children. Rare but serious side effects of lisdexamfetamine may include mania, sudden cardiac death in those with underlying heart problems, stimulant psychosis, and serotonin syndrome.
Interactions
- Acidifying agents: Drugs that acidify the urine, such as ascorbic acid, increase urinary excretion of dextroamphetamine, thus decreasing the half-life of dextroamphetamine in the body.
- Alkalinizing agents: Drugs that alkalinize the urine, such as sodium bicarbonate, decrease urinary excretion of dextroamphetamine, thus increasing the half-life of dextroamphetamine in the body.
- CYP2D6 inhibitors: Hydroxylation via the cytochrome P450 enzyme CYP2D6 is the major pathway of metabolism of dextroamphetamine. Potent CYP2D6 inhibitors, such as paroxetine, fluoxetine, bupropion, and duloxetine, among others, may inhibit the metabolism of dextroamphetamine and thereby increase exposure to it. Studies characterizing this potential interaction are currently lacking. Concomitant use of lisdexamfetamine with CYP2D6 inhibitors may increase the risk of serotonin syndrome due to greater drug exposure according to the FDA label for lisdexamfetamine.
- Monoamine oxidase inhibitors: Concomitant use of MAOIs and central nervous system stimulants such as lisdexamfetamine can cause a hypertensive crisis.
Pharmacology
Mechanism of action
|
via AADC |
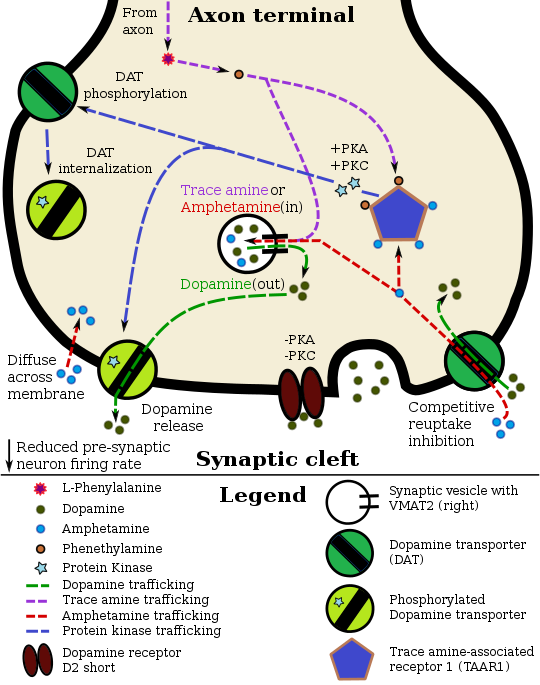
Lisdexamfetamine is an inactive prodrug that is converted in the body to dextroamphetamine, a pharmacologically active compound which is responsible for the drug's activity. After oral ingestion, lisdexamfetamine is broken down by enzymes in red blood cells to form L-lysine, a naturally occurring essential amino acid, and dextroamphetamine. The conversion of lisdexamfetamine to dextroamphetamine is not affected by gastrointestinal pH and is unlikely to be affected by alterations in normal gastrointestinal transit times.
The optical isomers of amphetamine, i.e., dextroamphetamine and levoamphetamine, are TAAR1 agonists and vesicular monoamine transporter 2 inhibitors that can enter monoamine neurons; this allows them to release monoamine neurotransmitters (dopamine, norepinephrine, and serotonin, among others) from their storage sites in the presynaptic neuron, as well as prevent the reuptake of these neurotransmitters from the synaptic cleft.
Lisdexamfetamine was developed with the goal of providing a long duration of effect that is consistent throughout the day, with reduced potential for abuse. The attachment of the amino acid lysine slows down the relative amount of dextroamphetamine available to the blood stream. Because no free dextroamphetamine is present in lisdexamfetamine capsules, dextroamphetamine does not become available through mechanical manipulation, such as crushing or simple extraction. A relatively sophisticated biochemical process is needed to produce dextroamphetamine from lisdexamfetamine. As opposed to Adderall, which contains roughly equal parts of racemic amphetamine and dextroamphetamine salts, lisdexamfetamine is a single-enantiomer dextroamphetamine formula. Studies conducted show that lisdexamfetamine dimesylate may have less abuse potential than dextroamphetamine and an abuse profile similar to diethylpropion at dosages that are FDA-approved for treatment of ADHD, but still has a high abuse potential when this dosage is exceeded by over 100%.
Pharmacokinetics

The oral bioavailability of amphetamine varies with gastrointestinal pH; it is well absorbed from the gut, and bioavailability is typically over 75% for dextroamphetamine. Amphetamine is a weak base with a pKa of 9.9; consequently, when the pH is basic, more of the drug is in its lipid soluble free base form, and more is absorbed through the lipid-rich cell membranes of the gut epithelium. Conversely, an acidic pH means the drug is predominantly in a water-soluble cationic (salt) form, and less is absorbed. Approximately 20% of amphetamine circulating in the bloodstream is bound to plasma proteins. Following absorption, amphetamine readily distributes into most tissues in the body, with high concentrations occurring in cerebrospinal fluid and brain tissue.
The half-lives of amphetamine enantiomers differ and vary with urine pH. At normal urine pH, the half-lives of dextroamphetamine and levoamphetamine are 9–11 hours and 11–14 hours, respectively. Highly acidic urine will reduce the enantiomer half-lives to 7 hours; highly alkaline urine will increase the half-lives up to 34 hours. The immediate-release and extended release variants of salts of both isomers reach peak plasma concentrations at 3 hours and 7 hours post-dose respectively. Amphetamine is eliminated via the kidneys, with 30–40% of the drug being excreted unchanged at normal urinary pH. When the urinary pH is basic, amphetamine is in its free base form, so less is excreted. When urine pH is abnormal, the urinary recovery of amphetamine may range from a low of 1% to a high of 75%, depending mostly upon whether urine is too basic or acidic, respectively. Following oral administration, amphetamine appears in urine within 3 hours. Roughly 90% of ingested amphetamine is eliminated 3 days after the last oral dose.
Lisdexamfetamine is a prodrug of dextroamphetamine. It is not as sensitive to pH as amphetamine when being absorbed in the gastrointestinal tract. Following absorption into the blood stream, lisdexamfetamine is completely converted by red blood cells to dextroamphetamine and the amino acid L-lysine by hydrolysis via undetermined aminopeptidase enzymes. This is the rate-limiting step in the bioactivation of lisdexamfetamine. The elimination half-life of lisdexamfetamine is generally less than 1 hour. Due to the necessary conversion of lisdexamfetamine into dextroamphetamine, levels of dextroamphetamine with lisdexamfetamine peak about one hour later than with an equivalent dose of immediate-release dextroamphetamine. Presumably due to its rate-limited activation by red blood cells, intravenous administration of lisdexamfetamine shows greatly delayed time to peak and reduced peak levels compared to intravenous administration of an equivalent dose of dextroamphetamine. The pharmacokinetics of lisdexamfetamine are similar regardless of whether it is administered orally, intranasally, or intravenously. Hence, in contrast to dextroamphetamine, parenteral use does not enhance the subjective effects of lisdexamfetamine. Because of its behavior as a prodrug and its pharmacokinetic differences, lisdexamfetamine has a longer duration of therapeutic effect than immediate-release dextroamphetamine and shows reduced misuse potential.
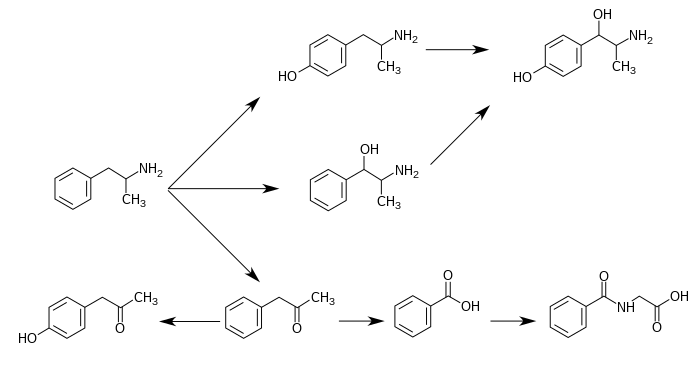
CYP2D6, dopamine β-hydroxylase (DBH), flavin-containing monooxygenase 3 (FMO3), butyrate-CoA ligase (XM-ligase), and glycine N-acyltransferase (GLYAT) are the enzymes known to metabolize amphetamine or its metabolites in humans. Amphetamine has a variety of excreted metabolic products, including 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, and phenylacetone. Among these metabolites, the active sympathomimetics are 4-hydroxyamphetamine,4-hydroxynorephedrine, and norephedrine. The main metabolic pathways involve aromatic para-hydroxylation, aliphatic alpha- and beta-hydroxylation, N-oxidation, N-dealkylation, and deamination. The known metabolic pathways, detectable metabolites, and metabolizing enzymes in humans include the following:
|
Metabolic pathways of amphetamine in humans
Para-
Hydroxylation Para-
Hydroxylation Para-
Hydroxylation unidentified
Beta-
Hydroxylation Beta-
Hydroxylation Oxidative
Deamination Oxidation
unidentified
Glycine
Conjugation |
Chemistry
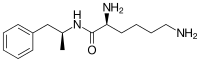
Lisdexamfetamine is a substituted amphetamine with an amide linkage formed by the condensation of dextroamphetamine with the carboxylate group of the essential amino acid L-lysine. The reaction occurs with retention of stereochemistry, so the product lisdexamfetamine exists as a single stereoisomer. There are many possible names for lisdexamfetamine based on IUPAC nomenclature, but it is usually named as N-[(2S)-1-phenyl-2-propanyl]-L-lysinamide or (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl]hexanamide. The condensation reaction occurs with loss of water:
-
(S)-PhCH
2CH(CH
3)NH
2 + (S)-HOOCCH(NH
2)CH
2CH
2CH
2CH
2NH
2 → (S,S)-PhCH
2CH(CH
3)NHC(O)CH(NH
2)CH
2CH
2CH
2CH
2NH
2 + H
2O
Amine functional groups are vulnerable to oxidation in air and so pharmaceuticals containing them are usually formulated as salts where this moiety has been protonated. This increases stability, water solubility, and, by converting a molecular compound to an ionic compound, increases the melting point and thereby ensures a solid product. In the case of lisdexamfetamine, this is achieved by reacting with two equivalents of methanesulfonic acid to produce the dimesylate salt, a water-soluble (792 mg mL−1) powder with a white to off-white color.
-
PhCH
2CH(CH
3)NHC(O)CH(NH
2)CH
2CH
2CH
2CH
2NH
2 + 2 CH
3SO
3H → [PhCH
2CH(CH
3)NHC(O)CH(NH+
3)CH
2CH
2CH
2CH
2NH+
3][CH
3SO−
3]
2
Comparison to other formulations
Lisdexamfetamine dimesylate is one marketed formulation delivering dextroamphetamine. The following table compares the drug to other amphetamine pharmaceuticals.
| drug | formula |
molar mass |
amphetamine base |
amphetamine base in equal doses |
doses with equal base content |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate | (C9H13N)2•H2SO4 |
368.49
|
270.41
|
73.38%
|
73.38%
|
—
|
22.0 mg
|
—
|
30.0 mg
|
|
| amphetamine sulfate | (C9H13N)2•H2SO4 |
368.49
|
270.41
|
73.38%
|
36.69%
|
36.69%
|
11.0 mg
|
11.0 mg
|
30.0 mg
|
|
| Adderall |
62.57%
|
47.49%
|
15.08%
|
14.2 mg
|
4.5 mg
|
35.2 mg
|
||||
| 25% | dextroamphetamine sulfate | (C9H13N)2•H2SO4 |
368.49
|
270.41
|
73.38%
|
73.38%
|
—
|
|||
| 25% | amphetamine sulfate | (C9H13N)2•H2SO4 |
368.49
|
270.41
|
73.38%
|
36.69%
|
36.69%
|
|||
| 25% | dextroamphetamine saccharate | (C9H13N)2•C6H10O8 |
480.55
|
270.41
|
56.27%
|
56.27%
|
—
|
|||
| 25% | amphetamine aspartate monohydrate | (C9H13N)•C4H7NO4•H2O |
286.32
|
135.21
|
47.22%
|
23.61%
|
23.61%
|
|||
| lisdexamfetamine dimesylate | C15H25N3O•(CH4O3S)2 |
455.49
|
135.21
|
29.68%
|
29.68%
|
—
|
8.9 mg
|
—
|
74.2 mg
|
|
| amphetamine base suspension | C9H13N |
135.21
|
135.21
|
100%
|
76.19%
|
23.81%
|
22.9 mg
|
7.1 mg
|
22.0 mg
|
|
History
Lisdexamfetamine was developed by New River Pharmaceuticals, who were bought by Takeda Pharmaceuticals through its acquisition of Shire Pharmaceuticals, shortly before it began being marketed. It was developed with the intention of creating a longer-lasting and less-easily abused version of dextroamphetamine, as the requirement of conversion into dextroamphetamine via enzymes in the red blood cells delays its onset of action, regardless of the route of administration.
On 23 April 2008, the FDA approved lisdexamfetamine for treatment of ADHD in adults. On 4 August 2009, Health Canada approved the marketing of 30 mg and 50 mg capsules of lisdexamfetamine for prescription use.
In January 2015, lisdexamfetamine was approved by the US Food and Drug Administration for treatment of binge eating disorder in adults.
The US Food and Drug Administration gave tentative approval to generic formulations of lisdexamfetamine in 2015. The expiration date for patent protection of lisdexamfetamine in the US was 24 February 2023. The Canadian patent expires 20 years from the filing date of 1 June 2004.
Production quotas for 2016 in the United States were 29,750 kg.
Society and culture
Names
Lisdexamfetamine is the International Nonproprietary Name (INN) and is a contraction of L-lysine-dextroamphetamine.
As of November 2020, lisdexamfetamine is sold under the following brand names: Aduvanz, Elvanse, Juneve, Lisdexamfetamine Dimesylate, Samexid, Tyvense, Venvanse, and Vyvanse.
Research
Depression
Some clinical trials that used lisdexamfetamine as an add-on therapy with a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) for treatment-resistant depression indicated that this is no more effective than the use of an SSRI or SNRI alone. Other studies indicated that psychostimulants potentiated antidepressants, and were under-prescribed for treatment resistant depression. In those studies, patients showed significant improvement in energy, mood, and psychomotor activity. Clinical guidelines advise caution in the use of stimulants for depression and advise them only as second- or third-line adjunctive agents.
In February 2014, Shire announced that two late-stage clinical trials had found that Vyvanse was not an effective treatment for depression, and development for this indication was discontinued. A 2018 meta-analysis of randomized controlled trials of lisdexamfetamine for antidepressant augmentation in people with major depressive disorder—the first to be conducted—found that lisdexamfetamine was not significantly better than placebo in improving Montgomery–Åsberg Depression Rating Scale scores, response rates, or remission rates. However, there was indication of a small effect in improving depressive symptoms that approached trend-level significance. Lisdexamfetamine was well-tolerated in the meta-analysis. The quantity of evidence was limited, with only four trials included. In a subsequent 2022 network meta-analysis, lisdexamfetamine was significantly effective as an antidepressant augmentation for treatment-resistant depression.
Although lisdexamfetamine has shown limited effectiveness in the treatment of depression in clinical trials, a phase II clinical study found that the addition of lisdexamfetamine to an antidepressant improved executive dysfunction in people with mild major depressive disorder but persisting executive dysfunction.
While development of lisdexamfetamine for major depressive disorder and bipolar depression was discontinued, the drug remains in phase II clinical trials for treatment of "mood disorders" as of October 2021.
External links
- "Lisdexamfetamine". Drug Information Portal. U.S. National Library of Medicine.
- "Lisdexamfetamine dimesylate". Drug Information Portal. U.S. National Library of Medicine.
| CNS stimulants | |
|---|---|
| Non-classical CNS stimulants |
|
|
α2-adrenoceptor agonists |
|
| Antidepressants | |
| Miscellaneous/others | |
| Related articles |
|
| DRAs |
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
|
||||||||||||||
| SRAs |
|
||||||||||||||
| Others |
|
||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
|
Catecholamines |
|
| Miscellaneous |
|