Pretomanid
 | |
| Clinical data | |
|---|---|
| Trade names | Dovprela |
| Other names | PA-824 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619056 |
| License data |
|
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
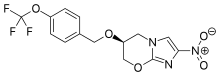
| Formula | C14H12F3N3O5 |
| Molar mass | 359.261 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pretomanid is an antibiotic medication used for the treatment of multi-drug-resistant tuberculosis affecting the lungs. It is generally used together with bedaquiline and linezolid. It is taken by mouth.
The most common side effects include nerve damage, acne, vomiting, headache, low blood sugar, diarrhea, and liver inflammation. It is in the nitroimidazole class of medications.
Pretomanid was approved for medical use in the United States in August 2019, and in the European Union in July 2020. Pretomanid was developed by TB Alliance. The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.
Medical uses
Pretomanid is indicated in combination with bedaquiline and linezolid, in adults, for the treatment of pulmonary extensively drug resistant (XDR), or treatment-intolerant or nonresponsive multidrug-resistant (MDR) tuberculosis (TB).
History
Pretomanid is the generic, nonproprietary name for the novel anti-bacterial drug compound formerly called PA-824. Pretomanid is referred to as "Pa" in regimen abbreviations, such as BPaL. The "preto" part of the compound's name honors Pretoria, South Africa, the home of a TB Alliance clinical development office where much of the drug's development took place, while the "-manid" stem designates compounds with similar chemical structures. This class of drug is variously referred to as nitroimidazoles or nitroimidazooxazines. Development of this compound was initiated because of the urgent need for new antibacterial drugs effective against resistant strains of tuberculosis. Also, current anti-TB drugs are mainly effective against replicating and metabolically active bacteria, creating a need for drugs effective against persisting or latent bacterial infections as often occur in patients with tuberculosis.
Discovery and pre-clinical development
Pretomanid was first identified in 2000, in a series of 100 nitroimidazopyran derivatives synthesized and tested for antitubercular activity, by PathoGenesis (now a subsidiary of Novartis). Importantly, pretomanid has activity against static M. tuberculosis isolates that survive under anaerobic conditions, with bactericidal activity comparable to that of the existing drug metronidazole. Pretomanid requires metabolic activation by Mycobacterium for antibacterial activity. Pretomanid was not the most potent compound in the series against cultures of M. tuberculosis, but it was the most active in infected mice after oral administration. Oral pretomanid was active against tuberculosis in mice and guinea pigs at safely tolerated dosages for up to 28 days.
Limited FDA approval
The U.S. Food and Drug Administration (FDA) approved pretomanid only in combination with bedaquiline and linezolid for treatment of a limited and specific population of adults with extensively drug resistant, treatment-intolerant or nonresponsive multidrug resistant pulmonary tuberculosis. Pretomanid was approved under the Limited Population Pathway (LPAD pathway) for antibacterial and antifungal drugs. The LPAD Pathway was established by Congress under the 21st Century Cures Act to expedite development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need. Pretomanid is only the third tuberculosis drug to receive FDA approval in more than 40 years.
The FDA granted Pretomanid priority review and orphan drug designation. The FDA granted The Global Alliance for TB Drug Development (TB Alliance) the approval of Pretomanid and a Tropical Disease Priority Review Voucher.
External links
- "Pretomanid". Drug Information Portal. U.S. National Library of Medicine.
| Nucleic acid inhibitor |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein synthesis inhibitor |
|
||||||||
| Cell envelope antibiotic |
|
||||||||
| Other/unknown | |||||||||
| Combinations | |||||||||
| |||||||||
