
T-1152
Подписчиков: 0, рейтинг: 0
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
270 μg/kg (subcutaneous, mice) 115 μg/kg (intraevenous, mice) 260 μg/kg (subcutaneous, rabbits) |
|
LDLo (lowest published)
|
2.5 mg/kg (oral, mice) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
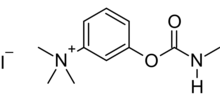
T-1152 is a quaternary carbamate anticholinesterase. It is synthesized by reaction of m-dimethylaminophenol with methyl isocyanate, followed by quaternization with methyl iodide. Since T-1152 is toxic by ingestion, it was patented as a rodenticide in 1932.
The chloride and methylsulfate salt of T-1152 is T-1690 (TL-1226) and AR-13, respectively.
See also
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood agents |
|
||||||||||||||
| Blister agents |
|
||||||||||||||
| Nerve agents |
|
||||||||||||||
| Neurotoxins |
|
||||||||||||||
| Nettle agents |
|
||||||||||||||
| Pulmonary/ choking agents |
|
||||||||||||||
| Vomiting agents | |||||||||||||||
| Incapacitating agents |
|
||||||||||||||
| Lachrymatory agents |
|
||||||||||||||
| Malodorant agents | |||||||||||||||
| Cornea-clouding agents | |||||||||||||||
| Biological toxins | |||||||||||||||
| Other |
|
||||||||||||||
|
Anticoagulants / Vitamin K antagonists |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Convulsants | |||||||||
| Calciferols | |||||||||
| Inorganic compounds | |||||||||
| Organochlorine | |||||||||
| Organophosphorus | |||||||||
| Carbamates | |||||||||
| Others | |||||||||