
Tanespimycin
| |
| Names | |
|---|---|
|
IUPAC name
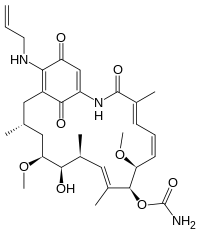
[(3S,5S,6R,7S,8E,10R,11S,12E,14E)-21-(allylamino)-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-8,12,14,18,21-pentaen-10-yl] carbamate
| |
| Other names
17-N-Allylamino-17-demethoxygeldanamycin
17-AAG | |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider |
|
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C31H43N3O8 | |
| Molar mass | 585.698 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tanespimycin (17-N-allylamino-17-demethoxygeldanamycin, 17-AAG) is a derivative of the antibiotic geldanamycin that is being studied in the treatment of cancer, specifically in younger patients with certain types of leukemia or solid tumors, especially kidney tumors.
It works by inhibiting Hsp90, which is expressed in those tumors.
It belongs to the family of drugs called antitumor antibiotics.
Clinical trials
Bristol-Myers Squibb conducted Phase 1 and Phase 2 clinical trials. However, in 2010 the company halted development of tanespimycin, during late-stage clinical trials as a potential treatment for multiple myeloma. While no definitive explanation was given, it has been suggested that Bristol-Myers Squibb halted development over concerns of the financial feasibility of tanespimycin development given the 2014 expiry of the patent on this compound, and the relative expense of manufacture.