
Toceranib
Подписчиков: 0, рейтинг: 0
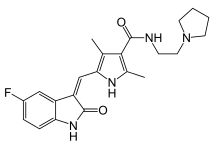 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Palladia |
| AHFS/Drugs.com | Veterinary Use |
| License data | |
| Routes of administration |
By mouth |
| Drug class | Antineoplastic agent |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 77% |
| Protein binding | 91%-93% |
| Elimination half-life | 16 h |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H25FN4O2 |
| Molar mass | 396.466 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Toceranib is a receptor tyrosine kinase inhibitor and is used in the treatment of canine mast cell tumor also called mastocytoma. Together with masitinib (Kinavet (US)/Masivet (EU/ROW) by AB Science), toceranib is the only dog-specific anti-cancer drug approved by the U.S. Food and Drug Administration (FDA). It is sold under the brand name Palladia as its phosphate salt, toceranib phosphate (INN) by Pfizer. It was developed by SUGEN as SU11654, a sister compound to sunitinib, which was later approved for human therapies. Toceranib is likely to act mostly through inhibition of the kit tyrosine kinase, though it may also have an anti-angiogenic effect.
External links
- "Toceranib". Drug Information Portal. U.S. National Library of Medicine.
| Angiopoietin |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CNTF |
|
||||||||||
| EGF (ErbB) |
|
||||||||||
| FGF |
|
||||||||||
| HGF (c-Met) |
|
||||||||||
| IGF |
|
||||||||||
| LNGF (p75NTR) |
|
||||||||||
| PDGF |
|
||||||||||
| RET (GFL) |
|
||||||||||
| SCF (c-Kit) |
|
||||||||||
| TGFβ |
|
||||||||||
| Trk |
|
||||||||||
| VEGF |
|
||||||||||
| Others |
|
||||||||||