
Trifluperidol
 | |
| Clinical data | |
|---|---|
| Trade names | Triperidol |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
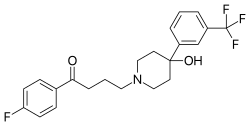
| Formula | C22H23F4NO2 |
| Molar mass | 409.425 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trifluperidol is a typical antipsychotic of the butyrophenone chemical class. It has general properties similar to those of haloperidol, but is considerably more potent by weight, and causes relatively more severe side effects, especially tardive dyskinesia and other extrapyramidal effects. It is used in the treatment of psychoses including mania and schizophrenia. It was discovered at Janssen Pharmaceutica in 1959.
Synthesis

The Grignard reaction between 1-benzyl-4-piperidone [3612-20-2] (1) and 3-bromobenzotrifluoride [401-78-5] (2) gives 1-benzyl-4-(3-(trifluoromethyl)phenyl)piperidin-4-ol, CID:12718203 (3). Catalytic hydrogenation removes the benzyl protecting group to give 4-[3-(trifluoromethyl)phenyl]-4-piperidinol [2249-28-7] (4). Alkylation with 4-Chloro-4'-fluorobutyrophenone [3874-54-2] (5) introduces the sidechain and hence completed the synthesis of Trifluperidol (6).