
Amoscanate
Подписчиков: 0, рейтинг: 0
| |
| |
| Names | |
|---|---|
|
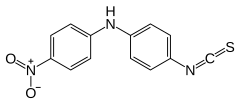
Preferred IUPAC name
4-Isothiocyanato-N-(4-nitrophenyl)aniline | |
| Other names
4-Isothiocyanato-4′-nitrodiphenylamine
Nithiocyamine | |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H9N3O2S | |
| Molar mass | 271.29 g·mol−1 |
| Melting point | 204 to 206 °C (399 to 403 °F; 477 to 479 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amoscanate (INN), also known as nithiocyamine, is an experimental anthelmintic agent of the aryl isothiocyanate class which was found to be highly effective in animals against the four major species of schistosomes which infect humans, and is also highly active against hookworm infection. However, significant liver toxicity was seen in lab animals at higher doses. The ether analogue of amoscanate, nitroscanate, is used in veterinary medicine as an anthelmintic.
Amoscanate was developed by Ciba.