
Aplaviroc
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
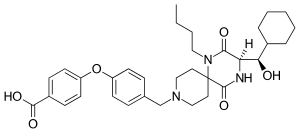
| Formula | C33H43N3O6 |
| Molar mass | 577.722 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Aplaviroc (INN, codenamed AK602 and GSK-873140) is a CCR5 entry inhibitor that belongs to a class of 2,5-diketopiperazines developed for the treatment of HIV infection. It was developed by GlaxoSmithKline.
In October 2005, all studies of aplaviroc were discontinued due to liver toxicity concerns. Some authors have claimed that evidence of poor efficacy may have contributed to termination of the drug's development; the ASCENT study, one of the discontinued trials, showed aplaviroc to be under-effective in many patients even at high concentrations.
See also
Further reading
- Horster S, Goebel FD (April 2006). "Serious doubts on safety and efficacy of CCR5 antagonists : CCR5 antagonists teeter on a knife-edge". Infection. 34 (2): 110–3. doi:10.1007/s15010-006-6206-1. PMID 16703305. S2CID 38463200.
| CC |
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CXC |
|
||||||||||||||||||||||||
| C (XC) |
|
||||||||||||||||||||||||
| CX3C |
|
||||||||||||||||||||||||
| Others |
|
||||||||||||||||||||||||