
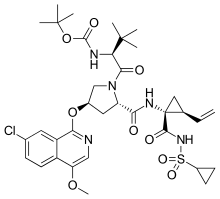
Asunaprevir
Подписчиков: 0, рейтинг: 0
| |
| Names | |
|---|---|
|
IUPAC name
3-Methyl-N-{[(2-methyl-2-propanyl)oxy]carbonyl}-L-valyl-(4R)-4-[(7-chloro-4-methoxy-1-isoquinolinyl)oxy]-N-{(1R,2S)-1-[(cyclopropylsulfonyl)carbamoyl]-2-vinylcyclopropyl}-L-prolinamide
| |
|
Systematic IUPAC name
tert-Butyl {(2S)-1-[(32S,34R,61R,62S)-17-chloro-62-ethenyl-14-methoxy-4,7,9,9-tetraoxo-2-oxa-9λ6-thia-5,8-diaza-1(1)-isoquinolina-3(4,2)-pyrrolidina-6(1,1),10(1)-dicyclopropadecaphan-31-yl]-3,3-dimethyl-1-oxobutan-2-yl}carbamate | |
| Other names
BMS-650032
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.206.482 |
| KEGG |
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C35H46ClN5O9S | |
| Molar mass | 748.29 g·mol−1 |
| Pharmacology | |
| J05AP06 (WHO) | |
| Legal status |
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Asunaprevir (formerly BMS-650032, brand name in Japan and RussiaSunvepra) is an experimental drug candidate for the treatment of hepatitis C. It was undergoing development by Bristol-Myers Squibb and has completed Phase III clinical trials in 2013.
Asunaprevir is an inhibitor of the hepatitis C virus enzyme serine protease NS3.Asunaprevir is being tested in combination with pegylated interferon and ribavirin, as well as in interferon-free regimens with other direct-acting antiviral agents including daclatasvir.
See also
| Hepatitis C |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hepatitis D | |||||||||
| Picornavirus | |||||||||
| Anti-influenza agents | |||||||||
| Multiple/general |
|
||||||||
| |||||||||