
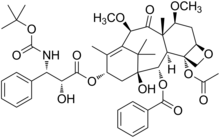
Cabazitaxel
 | |
| Clinical data | |
|---|---|
| Trade names | Jevtana |
| Other names | XRP-6258 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.205.741 |
| Chemical and physical data | |
| Formula | C45H57NO14 |
| Molar mass | 835.944 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.
Cabazitaxel was developed by Sanofi-Aventis and was approved by the U.S. Food and Drug Administration (FDA) for the treatment of hormone-refractory prostate cancer in June 2010. It is available as a generic medication.
Medical uses
Cabazitaxel is indicated in combination with prednisone for the treatment of metastatic castration-resistant prostate cancer following docetaxel-based treatment.
Mechanism of action
Taxanes enhance microtubule stabilization and inhibit cellular mitosis and division. Moreover, taxanes prevent androgen receptor (AR) signaling by binding cellular microtubules and the microtubule-associated motor protein dynein, thus averting AR nuclear translocation.
Clinical trials
In patients with metastatic castration-resistant prostate cancer (mCRPC), overall survival (OS) is markedly enhanced with cabazitaxel versus mitoxantrone after prior docetaxel treatment. FIRSTANA (ClinicalTrials.gov identifier: NCT01308567) assessed whether cabazitaxel 20 mg/m2 (C20) or 25 mg/m2 (C25) is superior to docetaxel 75 mg/m2 (D75) in terms of OS in patients with chemotherapy-naïve mCRPC. However, C20 and C25 did not demonstrate superiority for OS versus D75 in patients with chemotherapy-naïve mCRPC. Cabazitaxel and docetaxel demonstrated different toxicity profiles, and C20 showed the overall lowest toxicity. In a phase III trial with 755 men for the treatment of castration-resistant prostate cancer, median survival was 15.1 months for patients receiving cabazitaxel versus 12.7 months for patients receiving mitoxantrone. Cabazitaxel was associated with more grade 3–4 neutropenia (81.7%) than mitoxantrone (58%). Common adverse effects with cabazitaxel include neutropenia (including febrile neutropenia) and GIT side effects appeared mainly in diarrhea, whereas, neuropathy was rarely detected.
Pharmacokinetics
Cabazitaxel administration causes a decrease in plasma concentrations showing triphasic kinetics: a mean half life (t1/2) of 2.6 min in the first phase, a mean t1/2 of 1.3 h in the second phase, and a mean t1/2 of 77.3 h in the third phase.
Metabolism
Cabazitaxel is basically metabolized in the liver by [cytochrome P450 (CYP)3A4/5 > CYP2C8], which result in seven plasma metabolites and excreted 20 metabolites. During 14 days after administration, 80% of cabazitaxel is excreted: 76% in the feces and 3.7% as a renal excretion.
External links
- "Cabazitaxel". Drug Information Portal. U.S. National Library of Medicine.
- "Cabazitaxel Accord 20 mg/mL concentrate for solution infusion: Risk of medication errors and mix-up with Jevtana (60 mg/1.5 mL) solvent infusion". European Medicines Agency (EMA). October 28, 2020.
- Clinical trial number NCT00417079 for "XRP6258 Plus Prednisone Compared to Mitoxantrone Plus Prednisone in Hormone Refractory Metastatic Prostate Cancer (TROPIC)" at ClinicalTrials.gov
- Clinical trial number NCT01308580 for "Cabazitaxel at 20 mg/m² Compared to 25 mg/m² With Prednisone for the Treatment of Metastatic Castration Resistant Prostate Cancer (PROSELICA)" at ClinicalTrials.gov
- Clinical trial number NCT02485691 for "Cabazitaxel Versus the Switch to Alternative AR-targeted Agent (Enzalutamide or Abiraterone) in Metastatic Castration-resistant Prostate Cancer (mCRPC) Patients Previously Treated With Docetaxel and Who Rapidly Failed a Prior AR-targeted Agent (CARD)" at ClinicalTrials.gov