Caesium azide
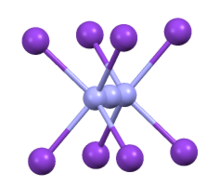 Coordination sphere of azide in CsN3
| |
| Names | |
|---|---|
|
IUPAC name
caesium azide
| |
| Other names
cesium azide
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.041.072 |
| EC Number |
|
|
PubChem CID
|
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CsN3 | |
| Molar mass | 174.926 g/mol |
| Appearance | colorless needles |
| Density | 3.5 g/cm3 |
| Melting point | 310 °C (590 °F; 583 K) |
| 224.2 g/100 mL (0 °C) | |
| Structure | |
| tetragonal | |
| I4/mcm, No. 140 | |
|
a = 6.5412 Å, c = 8.0908 Å
|
|
|
Formula units (Z)
|
4 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Caesium azide or cesium azide is an inorganic compound of caesium and nitrogen. It is a salt of azide with the formula CsN3.
Structure
CsN3 adopts the same structure as KN3, RbN3, and TlN3, crystallizing in a tetragonal distorted caesium chloride structure where each azide ion coordinates to eight metal cations, and each metal cation coordinates to eight terminal N centers. When heated to 151 °C, it transitions to a cubic structure.
Preparation and reactions
Caesium azide can be prepared from the neutralization reaction between hydrazoic acid and caesium hydroxide:
CsOH + HN3 → CsN3 + H2O
Caesium carbonate can also be used as the base:
Cs2CO3 + HN3 → CsN3 + CO2 + H2O
Caesium sulfate reacts with barium azide to form insoluble barium sulfate and caesium azide:
Cs2SO4 + Ba(N3)2 → 2CsN3 + BaSO4↓
The thermal decomposition of CsN3 in vacuo can be used as a method of generating high purity caesium metal:
2 CsN3 → 2 Cs + 3 N2
|
Caesium compounds
| |
|---|---|
|
Salts and covalent derivatives of the azide ion
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
