
Canertinib
Подписчиков: 0, рейтинг: 0
| |
| Names | |
|---|---|
|
Preferred IUPAC name
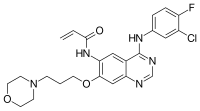
N-{4-(3-Chloro-4-fluoroanilino)-7-[3-(morpholin-4-yl)propoxy]quinazolin-6-yl}prop-2-enamide | |
| Other names
CI-1033; PD-183805
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H25ClFN5O3 | |
| Molar mass | 485.94 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Canertinib (CI-1033) is an experimental drug candidate for the treatment of cancer. It is an irreversible tyrosine-kinase inhibitor with activity against EGFR (IC50 0.8 nM), HER-2 (IC50 19 nM) and ErbB-4 (IC50 7 nM). By 2015, Pfizer had discontinued development of the drug.
Canertinib has been reported as a substrate for the transporter protein OATP1B3. Interaction of canertinib with OATP1B3 may alter its hepatic disposition and can lead to transporter mediated drug-drug interactions. Canertinib is not an inhibitor of the OATP1B1 or OATP1B3 transporters.