Carbon tetrachloride
|
| |||

| |||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Tetrachloromethane | |||
| Other names
Benziform
Benzinoform carbon(IV) chloride carbon tet Carboneum Tetrachloratum / Carbonei tetrachloridum Carboneum Chloratum / Carbonei chlorurum chloride of carbon CTC Freon-10 Halon-104 methane tetrachloride methyl tetrachloride Necatorina perchloromethane, PCM Refrigerant-10 Tetrachloretum Carbonicum Tetraform Tetrasol TCM | |||
| Identifiers | |||
|
|||
|
3D model (JSmol)
|
|||
| 1098295 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider |
|
||
| ECHA InfoCard | 100.000.239 | ||
| EC Number |
|
||
| 2347 | |||
| KEGG |
|
||
|
PubChem CID
|
|||
| RTECS number |
|
||
| UNII | |||
| UN number | 1846 | ||
|
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CCl4 | |||
| Molar mass | 153.81 g/mol | ||
| Appearance | Colourless liquid | ||
| Odor | Sweet, chloroform-like odor | ||
| Density |
|
||
| Melting point | −22.92 °C (−9.26 °F; 250.23 K) | ||
| Boiling point | 76.72 °C (170.10 °F; 349.87 K) | ||
|
|||
| Solubility | Soluble in alcohol, ether, chloroform, benzene, naphtha, CS2, formic acid | ||
| log P | 2.64 | ||
| Vapor pressure | 11.94 kPa at 20 °C | ||
|
Henry's law
constant (kH) |
2.76×10−2 atm·m3/mol | ||
| −66.60×10−6 cm3/mol | |||
| Thermal conductivity | 0.1036 W/m·K (300 K) | ||
|
Refractive index (nD)
|
1.4607 | ||
| Viscosity | 0.86 mPa·s | ||
| 0 D | |||
| Structure | |||
| Monoclinic | |||
| Tetragonal | |||
| Tetrahedral | |||
| 0 D | |||
| Thermochemistry | |||
|
Heat capacity (C)
|
132.6 J/mol·K | ||
|
Std molar
entropy (S⦵298) |
214.39 J/mol·K | ||
|
Std enthalpy of
formation (ΔfH⦵298) |
−95.6 kJ/mol | ||
|
Gibbs free energy (ΔfG⦵)
|
−87.34 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
|
Main hazards
|
potential occupational carcinogen | ||
| GHS labelling: | |||
  
|
|||
| Danger | |||
| H301, H302, H311, H331, H351, H372, H412, H420 | |||
| P201, P202, P260, P261, P264, P270, P271, P273, P280, P281, P301+P310, P302+P352, P304+P340, P308+P313, P311, P312, P314, P321, P322, P330, P361, P363, P403+P233, P405, P501, P502 | |||
| NFPA 704 (fire diamond) | |||
| 982 °C (1,800 °F; 1,255 K) | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
250 mg/kg | ||
|
LC50 (median concentration)
|
|
||
|
LCLo (lowest published)
|
|
||
| NIOSH (US health exposure limits): | |||
|
PEL (Permissible)
|
TWA 10 ppm C 25 ppm 200 ppm (5-minute maximum peak in any 4 hours) | ||
|
REL (Recommended)
|
Ca ST 2 ppm (12.6 mg/m3) [60-minute] | ||
|
IDLH (Immediate danger)
|
200 ppm | ||
| Safety data sheet (SDS) | ICSC 0024 | ||
| Related compounds | |||
|
Other anions
|
Carbon tetrafluoride Carbon tetrabromide Carbon tetraiodide |
||
|
Other cations
|
Silicon tetrachloride Germanium tetrachloride Tin tetrachloride Lead tetrachloride |
||
|
Related chloromethanes
|
Chloromethane Dichloromethane Chloroform |
||
| Supplementary data page | |||
| Carbon tetrachloride (data page) | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also recognised by the IUPAC) is a chemical compound with the chemical formula CCl4. It is a non-flammable, colourless liquid with a "sweet" chloroform-like smell that can be detected at low levels. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal.
Tradenames include; carbon tet, Thawpit and Benzinoform in the cleaning industry, Halon-104 in firefighting, Refrigerant-10 in HVACR, and Necatorina as a medication.
Properties
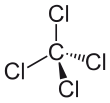
In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetric geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane. As a solvent, it is well suited to dissolving other non-polar compounds such as fats and oils. It can also dissolve iodine. It is volatile, giving off vapors with a smell characteristic of other chlorinated solvents, somewhat similar to the tetrachloroethylene smell reminiscent of dry cleaners' shops.
Solid tetrachloromethane has two polymorphs: crystalline II below −47.5 °C (225.6 K) and crystalline I above −47.5 °C. At −47.3 °C it has monoclinic crystal structure with space group C2/c and lattice constants a = 20.3, b = 11.6, c = 19.9 (.10−1 nm), β = 111°.
With a specific gravity greater than 1, carbon tetrachloride will be present as a dense nonaqueous phase liquid if sufficient quantities are spilled in the environment.
History and synthesis
Carbon tetrachloride was originally synthesized by Michael Faraday who named it "protochloride of carbon" in 1820 via decomposition of hexachloroethane ("perchloride of carbon") which he synthesized by chlorination of ethylene. The protochloride of carbon has been previously misidentified as tetrachloroethylene due to being made with the same reaction of hexachloroethane. Later in the 19th century, the name protochloride of carbon was used for tetrachloroethylene and carbon tetrachloride was called "bichloride of carbon".
Prior to the 1950s, carbon tetrachloride was manufactured by the chlorination of carbon disulfide at 105 to 130 °C:
But now it is mainly produced from methane:
- CH4 + 4 Cl2 → CCl4 + 4 HCl
The production often utilizes by-products of other chlorination reactions, such as from the syntheses of dichloromethane and chloroform. Higher chlorocarbons are also subjected to this process named "chlorinolysis":
- C2Cl6 + Cl2 → 2 CCl4
The production of carbon tetrachloride has steeply declined since the 1980s due to environmental concerns and the decreased demand for CFCs, which were derived from carbon tetrachloride. In 1992, production in the U.S./Europe/Japan was estimated at 720,000 tonnes.
Safety
At high temperatures in air, it decomposes or burns to produce poisonous phosgene. This was a common problem when carbon tetrachloride was used as a fire extinguisher, there have been deaths due to its conversion to phosgene reported.
Carbon tetrachloride is a suspected human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. The World Health Organization reports carbon tetrachloride can induce hepatocellular carcinomas (hepatomas) in mice and rats. The doses inducing hepatic tumours are higher than those inducing cell toxicity. The International Agency for Research on Cancer (IARC) classified this compound in Group 2B, "possibly carcinogenic to humans". Carbon tetrachloride is one of the most potent hepatotoxins (toxic to the liver), so much so that it is widely used in scientific research to evaluate hepatoprotective agents. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system and degenerate the liver and kidneys, and prolonged exposure may lead to coma or death. Chronic exposure to carbon tetrachloride can cause liver and kidney damage and could result in cancer. See safety data sheets.
The effects of carbon tetrachloride on human health and the environment have been assessed under REACH in 2012 in the context of the substance evaluation by France.
In 2008, a study of common cleaning products found the presence of carbon tetrachloride in "very high concentrations" (up to 101 mg/m3) as a result of manufacturers' mixing of surfactants or soap with sodium hypochlorite (bleach).
Carbon tetrachloride is also both ozone-depleting and a greenhouse gas. However, since 1992 its atmospheric concentrations have been in decline for the reasons described above (see atmospheric concentration graphs in the gallery). CCl4 has an atmospheric lifetime of 85 years.
Uses
In organic chemistry, carbon tetrachloride serves as a source of chlorine in the Appel reaction.
Carbon tetrachloride made from heavy chlorine-37 has been used in the detection of neutrinos.
One specialty use of carbon tetrachloride is in stamp collecting, to reveal watermarks on postage stamps without damaging them. A small amount of the liquid is placed on the back of a stamp, sitting in a black glass or obsidian tray. The letters or design of the watermark can then be seen clearly.
Historical uses
Carbon tetrachloride was widely used as a dry cleaning solvent, as a refrigerant, and in lava lamps. In the last case, carbon tetrachloride is a key ingredient that adds weight to the otherwise buoyant wax.
Medical uses
Carbon tetrachloride has been briefly used as a volatile inhalation anaesthetic and analgesic for intense menstruation pains and headaches in the mid-19th century. It was introduced as a safer alternative to Chloroform in 1864. In December 1865, the Scottish obstetrician who discovered the anaesthetic effects of chloroform on humans, James Young Simpson had experimented with carbon tetrachloride as an anaesthetic. Simpson named the compound "Chlorocarbon" for its similarity to chloroform. His experiments included injecting carbon tetrachloride into two women's vaginas. Simpson orally consumed carbon tetrachloride and described it as having "the same effect as swallowing a capsule of chloroform".
Because of the higher amount of chlorine atoms (compared to chloroform) in its molecule, carbon tetrachloride has a stronger anaesthetic effect than chloroform and required a smaller amount. It is less volatile than chloroform, therefore it was more difficult to apply and needed warm water to evaporate. Its smell has been described as "fruity" and "more pleasant than chloroform" and had a "pleasant taste". Carbon tetrachloride for anaesthetic use was made by the chlorination of carbon disulphide. It was used on at least 50 patients, of which most were women in labour. Such use was experimental and the anaesthetic use of carbon tetrachloride never gained popularity.
Beginning in 1922, capsules of pure carbon tetrachloride were marketed by Merck under the name Necatorina (variants include Neo-necatorina and Necatorine). Necatorina was used as a medication against parasitic diseases in humans. This medication was most prevalently used in Latin American countries. Its toxicity was not well-understood at the time and toxic effects were attributed to impurities in the capsules rather than carbon tetrachloride itself.
Solvent
It once was a popular solvent in organic chemistry, but because of its adverse health effects, it is rarely used today. It is sometimes useful as a solvent for infrared spectroscopy, because there are no significant absorption bands above 1600 cm−1. Because carbon tetrachloride does not have any hydrogen atoms, it was historically used in proton NMR spectroscopy. In addition to being toxic, its dissolving power is low. Its use in NMR spectroscopy has been largely superseded by deuterated solvents (mainly deuterochloroform). Use of carbon tetrachloride in determination of oil has been replaced by various other solvents, such as tetrachloroethylene. Because it has no C–H bonds, carbon tetrachloride does not easily undergo free-radical reactions. It is a useful solvent for halogenations either by the elemental halogen or by a halogenation reagent such as N-bromosuccinimide (these conditions are known as Wohl–Ziegler bromination).
Fire suppression
In 1910, the Pyrene Manufacturing Company of Delaware filed a patent to use carbon tetrachloride to extinguish fires. The liquid was vaporized by the heat of combustion and extinguished flames, an early form of gaseous fire suppression. At the time it was believed the gas simply displaced oxygen in the area near the fire, but later research found that the gas actually inhibits the chemical chain reaction of the combustion process.
In 1911, Pyrene patented a small, portable extinguisher that used the chemical. The extinguisher consisted of a brass bottle with an integrated hand-pump that was used to expel a jet of liquid toward the fire. As the container was unpressurized, it could easily be refilled after use. Carbon tetrachloride was suitable for liquid and electrical fires and the extinguishers were often carried on aircraft or motor vehicles. However, as early as 1920, there were reports of fatalities caused by the chemical when used to fight a fire in a confined space.
In the first half of the 20th century, another common fire extinguisher was a single-use, sealed glass globe known as a "fire grenade", filled with either carbon tetrachloride or salt water. The bulb could be thrown at the base of the flames to quench the fire. The carbon tetrachloride type could also be installed in a spring-loaded wall fixture with a solder-based restraint. When the solder melted by high heat, the spring would either break the globe or launch it out of the bracket, allowing the extinguishing agent to be automatically dispersed into the fire.
A well-known brand of fire grenade was the "Red Comet", which was variously manufactured with other fire-fighting equipment in the Denver, Colorado area by the Red Comet Manufacturing Company from its founding in 1919 until manufacturing operations were closed in the early 1980s.
Refrigerants
Prior to the Montreal Protocol, large quantities of carbon tetrachloride were used to produce the chlorofluorocarbon refrigerants R-11 (trichlorofluoromethane) and R-12 (dichlorodifluoromethane). However, these refrigerants play a role in ozone depletion and have been phased out. Carbon tetrachloride is still used to manufacture less destructive refrigerants.
Fumigant
Carbon tetrachloride was widely used as a fumigant to kill insect pests in stored grain. It was employed in a mixture known as 80/20, that was 80% carbon tetrachloride and 20% Carbon disulfide. The United States Environmental Protection Agency banned its use in 1985.
Gallery
CCl4 measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion.
External links
- International Chemical Safety Card 0024
- NIOSH Pocket Guide to Chemical Hazards. "#0107". National Institute for Occupational Safety and Health (NIOSH).
- "Carbon Tetrachloride (Group 2B)". International Agency for Research on Cancer (IARC) – Summaries & Evaluations. 71: 401. 1999.
- IARC Monograph: "Carbon Tetrachloride"
- Toxicological profile for carbon tetrachloride
- Environmental health criteria for carbon tetrachloride
- Carbon tetrachloride MSDS at Hazardous Chemical Database
- Substance profile at ntp.niehs.nih.gov
- ChemSub Online: Carbon tetrachloride
|
Inorganic compounds of carbon and related ions
| |
|---|---|
| Compounds | |
| Carbon ions |
|
| Nanostructures | |
| Oxides and related | |