
Certolizumab pegol
Certolizumab pegol
Подписчиков: 0, рейтинг: 0
 | |
 Syringe with 200mg Certolizumab pegol
| |
| Monoclonal antibody | |
|---|---|
| Type | Fab' fragment |
| Source | Humanized (from mouse) |
| Target | TNF alpha |
| Clinical data | |
| Trade names | Cimzia |
| Other names | CDP870 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608041 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | about 11 days |
| Excretion | Kidney (PEG only) |
| Identifiers | |
| CAS Number | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| Chemical and physical data | |
| Formula | C2115H3252N556O673S16 |
| Molar mass | 47749.46 g·mol−1 |
|
| |
Certolizumab pegol, sold under the brand name Cimzia, is a biopharmaceutical medication for the treatment of Crohn's disease,rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.
It is on the World Health Organization's List of Essential Medicines.
Medical uses
- Crohn's Disease
- On April 22, 2008, the U.S. Food and Drug Administration (FDA) approved Cimzia for the treatment of Crohn's disease in people who did not respond sufficiently or adequately to standard therapy.
- Rheumatoid arthritis
- On June 26, 2009, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a positive opinion recommending that the European Commission grant a marketing authorisation for Cimzia for the treatment of rheumatoid arthritis only - the CHMP refused approval for the treatment of Crohn's disease. The marketing authorisation was granted to UCB Pharma SA on October 1, 2009.
- Psoriatic arthritis
- On September 27, 2013, the U.S. FDA approved Cimzia for the treatment of adult patients with active psoriatic arthritis.
Method of action
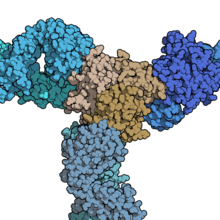
Three certolizumab molecules (blue) binding a homotrimer of TNF-alpha (tan). Certolizumab can block TNF in both its soluble form (freely circulating in the bloodstream) and its transmembrane form (bound to the membrane of a cell). From PDB: 5WUX.
Certolizumab pegol is a monoclonal antibody directed against tumor necrosis factor alpha. More precisely, it is a PEGylated Fab' fragment of a humanized TNF inhibitor monoclonal antibody.
Clinical trials
- Crohn's disease
- Positive results have been demonstrated in two phase III trials (PRECiSE 1 and 2) of certolizumab pegol versus placebo in moderate to severe active Crohn's disease.
- Axial spondyloarthritis
- In 2013, a phase 3 double blind randomized placebo-controlled study found significantly positive results in patient self-reported questionnaires, with rapid improvement of function and pain reduction, in patients with axial spondyloarthritis.
- Rheumatoid arthritis
- Certolizumab appears beneficial in those with rheumatoid arthritis.
Side effects
Significant side effects occur in 2% of people who take the medication.
External links
- "Certolizumab pegol". Drug Information Portal. U.S. National Library of Medicine.
- certolizumab+pegol at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
| Intracellular (initiation) |
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intracellular (reception) |
|
||||||||||||||
| Extracellular |
|
||||||||||||||
| Unsorted | |||||||||||||||