
Cordycepin
| |
| Names | |
|---|---|
|
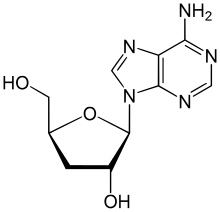
IUPAC name
3′-Deoxyadenosine
| |
|
Systematic IUPAC name
(2S,3R,5S)-2-(6-Amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolan-3-ol | |
| Other names
Cordycepine
9-(3-Deoxy-β-D-ribofuranosyl)adenine 3-dA | |
| Identifiers | |
|
|
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.000.720 |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H13N5O3 | |
| Molar mass | 251.246 g·mol−1 |
| Melting point | 225.5 °C (437.9 °F; 498.6 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cordycepin, or 3'-deoxyadenosine, is a derivative of the nucleoside adenosine, differing from the latter by the replacement of the hydroxy group in the 3' position with a hydrogen. It was initially extracted from the fungus Cordyceps militaris, but can now be produced synthetically. It is also found in other Cordyceps species as well as Ophiocordyceps sinensis.
Cordycepin is produced in cordyceps as a means of infecting insect populations, due to cordycepin's biological activity
Because cordycepin is similar to adenosine, some enzymes cannot discriminate between the two. It can therefore participate in certain biochemical reactions (for example, 3-dA can trigger the premature termination of mRNA synthesis). By acting as an adenosine analog, cordycepin was found to be the most potent molecular circadian clock resetter out of several screened compounds.
Cordycepin has displayed cytotoxicity against some leukemic cell lines in vitro. Additionally, cordycepin has been shown to display an effect in some types of other cancers, such as lung, renal, colon, and breast cancer. Cordycepin has been shown to reduce viable A549 lung cancer cell populations by 50%.
Cordycepin has been found to produce rapid, robust imipramine-like antidepressant effects in animal models of depression, and these effects, similarly to those of imipramine, are dependent on enhancement of AMPA receptor signaling.
Cordycepin has been shown to have anti-inflammatory qualities, as well as the ability to defend against injury from cerebral ischemia in mice.