
Dactolisib
| |
| |
| Names | |
|---|---|
|
Preferred IUPAC name
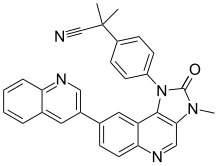
2-Methyl-2-{4-[3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydro-1H-imidazo[4,5-c]quinolin-1-yl]phenyl}propanenitrile | |
| Other names
NVP-BEZ235; BEZ-235; RTB101
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider |
|
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H23N5O | |
| Molar mass | 469.548 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dactolisib (codenamed NVP-BEZ235 and BEZ-235, also known as RTB101) is an imidazoquinoline derivative acting as a PI3K inhibitor. It also inhibits mTOR. It is being investigated as a possible cancer treatment.
It has been shown to be toxic to Waldenström's macroglobulinemia cells.
It was the first PI3K inhibitor to enter clinical trials, in 2006.
A phase IB/II clinical trial for locally advanced or metastatic HER2 negative breast cancer has completed.
A phase II clinical trial for advanced pancreatic neuroendocrine tumors (pNET) had initially reported results, but was later terminated because insufficient normal tissue tolerance to the drug. A phase I clinical trial of BEZ235 in patients with advanced renal cell carcinoma had to be terminated prematurely due to toxicity and a lack of clinical efficacy . Another Phase Ib study on patients with various solid cancers found severe normal tissue toxicity as well when BEZ235/Dactolisib was administered in combination with the mTOR inhibitor Everolimus. The authors concluded that the combination of both drugs demonstrated limited efficacy and tolerance. BEZ235 systemic exposure increased in a dose-proportional manner while oral bioavailability was quite low, which may be related to gastrointestinal-specific toxicity . A phase I study of BEZ-235 to treat acute lymphoid leukaemia was initiated in 2012, but no results were published since then.
A phase 2a randomized, placebo-controlled clinical trial published in 2018 showed that everolimus in combination with dactolisib decreased the rate of reported infections in an elderly population.