
Damnacanthal
Подписчиков: 0, рейтинг: 0
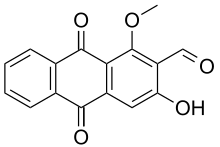
| |
| Names | |
|---|---|
|
Preferred IUPAC name
3-Hydroxy-1-methoxy-9,10-dioxo-9,10-dihydroanthracene-2-carbaldehyde | |
| Other names
3-Hydroxy-1-methoxyanthraquinone-2-aldehyde
| |
| Identifiers | |
|
|
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.208.625 |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H10O5 | |
| Molar mass | 282.251 g·mol−1 |
| Density | 1.461 g/mL |
| Boiling point | 532 °C (990 °F; 805 K) |
| Related compounds | |
|
Related arylformaldehydes
|
Gossypol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Damnacanthal is an anthraquinone isolated from the root of Morinda citrifolia, using water or organic solvents.
Pharmacology
In a 1995 in vitro study, damnacanthal was found to act as a potent and selective inhibitor of p56lck tyrosine kinase.
|
Types of natural anthraquinones
| |
|---|---|
| Dihydroxyanthraquinones | |
| Trihydroxyanthraquinones | |
| Misc: | |