
Dihexa
 | |
| Clinical data | |
|---|---|
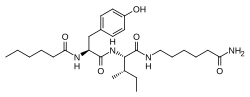
| Other names | N-(1-Oxohexyl)-l-tyrosyl-N-(6-amino-6-oxohexyl)-l-isoleucinamide |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H44N4O5 |
| Molar mass | 504.672 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dihexa (developmental code name PNB-0408), also known as N-hexanoic-Tyr-Ile-(6) aminohexanoic amide, is an oligopeptide drug derived from angiotensin IV that binds with high affinity to hepatocyte growth factor (HGF) and potentiates its activity at its receptor, c-Met. The compound has been found to potently improve cognitive function in animal models of Alzheimer's disease-like mental impairment. In an assay of neurotrophic activity, Dihexa was found to be seven orders of magnitude more potent than brain-derived neurotrophic factor.
According to a patent, "Short duration safety studies with Dihexa have uncovered no apparent toxicity. Of particular note is a lack of neoplastic induction, since c-Met is recognized as an oncogene. This is unsurprising since oncogenesis requires multiple mutations including both oncogene induction and tumor suppressor attenuation."
History
Dihexa was developed by Joseph Harding and his team at Washington State University. Later developments were done under "M3 Biotechnology", a company founded to commercialise Dihexa.
| AChE inhibitor medications | |
|---|---|
| Other medications | |
| Experimental BACE inhibitors | |