Morpheein
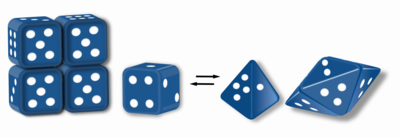
Morpheeins are proteins that can form two or more different homo-oligomers (morpheein forms), but must come apart and change shape to convert between forms. The alternate shape may reassemble to a different oligomer. The shape of the subunit dictates which oligomer is formed. Each oligomer has a finite number of subunits (stoichiometry). Morpheeins can interconvert between forms under physiological conditions and can exist as an equilibrium of different oligomers. These oligomers are physiologically relevant and are not misfolded protein; this distinguishes morpheeins from prions and amyloid. The different oligomers have distinct functionality. Interconversion of morpheein forms can be a structural basis for allosteric regulation, an idea noted many years ago, and later revived. A mutation that shifts the normal equilibrium of morpheein forms can serve as the basis for a conformational disease. Features of morpheeins can be exploited for drug discovery. The dice image (Fig 1) represents a morpheein equilibrium containing two different monomeric shapes that dictate assembly to a tetramer or a pentamer. The one protein that is established to function as a morpheein is porphobilinogen synthase, though there are suggestions throughout the literature that other proteins may function as morpheeins (for more information see "Table of Putative Morpheeins" below).
Implications for drug discovery
Conformational differences between subunits of different oligomers and related functional differences of a morpheein provide a starting point for drug discovery. Protein function is dependent on the oligomeric form; therefore, the protein's function can be regulated by shifting the equilibrium of forms. A small molecule compound can shift the equilibrium either by blocking or favoring formation of one of the oligomers. The equilibrium can be shifted using a small molecule that has a preferential binding affinity for only one of the alternate morpheein forms. An inhibitor of porphobilinogen synthase with this mechanism of action has been documented.
Implications for allosteric regulation
The morpheein model of allosteric regulation has similarities to and differences from other models. The concerted model (the Monod, Wyman and Changeux (MWC) model) of allosteric regulation requires all subunits to be in the same conformation or state within an oligomer like the morpheein model. However, neither this model nor the sequential model (Koshland, Nemethy, and Filmer model) takes into account that the protein may dissociate to interconvert between oligomers. Nonetheless, shortly after these theories were described, two groups of workers proposed what is now called the morpheein model and showed that it accounted for the regulatory behavior of glutamate dehydrogenase. Kurganov and Friedrich discussed models of this kind extensively in their books.
Implications for teaching about protein structure-function relationships
It is generally taught that a given amino acid sequence will have only one physiologically relevant (native) quaternary structure; morpheeins challenge this concept. The morpheein model does not require gross changes in the basic protein fold. The conformational differences that accompany conversion between oligomers may be similar to the protein motions necessary for function of some proteins. The morpheein model highlights the importance of conformational flexibility for protein functionality and offers a potential explanation for proteins showing non-Michaelis-Menten kinetics, hysteresis, and/or protein concentration dependent specific activity.
Implications for understanding the structural basis for disease
The term "conformational disease" generally encompasses mutations that result in misfolded proteins that aggregate, such as Alzheimer's and Creutzfeldt–Jakob diseases. In light of the discovery of morpheeins, however, this definition could be expanded to include mutations that shift an equilibrium of alternate oligomeric forms of a protein. An example of such a conformational disease is ALAD porphyria, which results from a mutation of porphobilinogen synthase that causes a shift in its morpheein equilibrium.
Table of proteins whose published behavior is consistent with that of a morpheein
| Protein | Example species | EC number | CAS number | Alternate oligomers | Evidence |
|---|---|---|---|---|---|
| Acetyl-CoA carboxylase-1 | Gallus domesticus | EC 6.4.1.2 | 9023-93-2 | inactive dimer, active dimer, larger | Effector molecules impact multimerization, Multiple/protein moonlighting functions |
| α-Acetylgalactosaminidase | Bos taurus | EC 4.3.2.2 | 9027-81-0 | inactive monomer, active tetramer | Substrate binding/turnover impacts multimerization, Protein concentration dependent specific activity, Different assemblies have different activities, Conformationally distinct oligomeric forms. |
| Adenylosuccinate lyase | Bacillus subtilis | EC 4.3.2.2 | 9027-81-0 | monomer, dimer, trimer, tetramer | Mutations shift the equilibrium of oligomers, Oligomer-dependent kinetic parameters, Protein concentration dependent molecular weight |
| Aristolochene synthase | Penicillium roqueforti | EC 4.2.3.9 | 94185-89-4 | monomer, higher order | Protein concentration dependent specific activity |
| L-Asparaginase | Leptosphaeria michotii | EC 3.5.1.1 | 9015-68-3 | dimer, tetramer, inactive octamer | Substrate binding/turnover impacts multimerization |
| Aspartokinase | Escherichia coli | EC 2.7.2.4 & EC 1.1.1.3 | 9012-50-4 | monomer, dimer, tetramer | Multiple/protein moonlighting functions, Conformationally distinct oligomeric forms |
| ATPase of the ABCA1 transporter | Homo sapiens | dimer, tetramer | Substrate binding/turnover impacts multimerization | ||
| Biotin—(acetyl-CoA-carboxylase) ligase holoenzyme synthetase | Escherichia coli | EC 6.3.4.15 | 37340-95-7 | monomer, dimer | Multiple/protein moonlighting functions, Different assemblies have different activities |
| Chorismate mutase | Escherichia coli | EC 5.4.99.5 | 9068-30-8 | dimer, trimer, hexamer | Conformationally distinct oligomeric forms |
| Citrate synthase | Escherichia coli | EC 2.3.3.1 | 9027-96-7 | monomer, dimer, trimer, tetramer, pentamer, hexamer, dodecamer | Substrate binding/turnover impacts multimerization, Characterized equilibrium of oligomers, Protein concentration dependent specific activity, pH-dependent oligomeric equilibrium |
| Cyanovirin-N | Nostoc ellipsosporum | 918555-82-5 | monomer and domain-swapped dimer | Characterized equilibrium of oligomers, Conformationally distinct oligomeric forms | |
| 3-oxoacid CoA-transferase | Sus scrofa domestica | EC 2.8.3.5 | 9027-43-4 | dimer, tetramer | Chromatographically separable oligomers, Substrate might preferentially stabilize one form |
| Cystathionine β-synthase | Homo sapiens | EC 4.2.1.22 | 9023-99-8 | multiple - ranges from dimer to 16-mer | Effector molecules impact multimerization, Mutations shift the equilibrium of oligomers, Different assemblies have different activities, disease-causing mutations at sites distant from active site |
| D-amino acid oxidase | EC 1.4.3.3 | 9000-88-8 | monomers, dimers, higher-order oligomers | Oligomer-dependent kinetic parameters | |
| Dihydrolipoamide dehydrogenase | Sus scrofa domestica | EC 1.8.1.4 | 9001-18-7 | monomer, two different dimer forms, tetramer | Multiple/protein moonlighting functions, Different assemblies have different activities, pH-dependent oligomeric equilibrium, Conformationally distinct oligomeric forms |
| Dopamine β-monooxygenase | Bos taurus | EC 1.14.17.1 | 9013-38-1 | dimers, tetramers | Effector molecules impact multimerization, Characterized equilibrium of oligomers, Oligomer-dependent kinetic parameters |
| Geranylgeranyl pyrophosphate synthase / Farnesyltranstransferase | Homo sapiens | EC 2.5.1.29 | 9032-58-0 | hexamer, octamer | Effector molecules impact multimerization |
| GDP-mannose 6-dehydrogenase | Pseudomonas aeruginosa | EC 1.1.1.132 | 37250-63-8 | trimer, 2 tetramers, and hexamer | Protein concentration dependent specific activity, Kinetic hysteresis |
| Glutamate dehydrogenase | Bos taurus | EC 1.4.1.2 | 9001-46-1 | active & inactive hexamers, higher order | Effector molecules impact multimerization, Characterized equilibrium of oligomers |
| Glutamate racemase | Mycobacterium tuberculosis, Escherichia coli, Bacillus subtilis, Aquifex pyrophilus | EC 5.1.1.3 | 9024-08-02 | monomer, 2 dimers, tetramer | Multiple/protein moonlighting functions, Characterized equilibrium of oligomers, Conformationally distinct oligomeric forms |
| Glyceraldehyde-3-phosphate dehydrogenase | Oryctolagus cuniculas, Sus scrofa domestica | EC 1.2.1.12 1.2.1.12 | 9001-50-7 | monomer, dimer, tetramer Characterized equilibrium of oligomers, Different assemblies have different activities | |
| Glycerol kinase | Escherichia coli | EC 2.7.1.30 | 9030-66-4 | monomer and 2 tetramers | Characterized equilibrium of oligomers, Conformationally distinct oligomeric forms, Effector functions by preventing domain motion |
| HIV-Integrase | Human immunodeficiency virus-1 | EC 2.7.7.- | monomer, dimer, tetramer, higher order | Effector molecules impact multimerization, Multiple/protein moonlighting functions, Different assemblies have different activities | |
| HPr-Kinase/phosphatase | Bacillus subtilis, Lactobacillus casei, Mycoplasma pneumoniae, Staphylococcus xylosus | EC 2.7.1.-/ EC 3.1.3.- | 9026-43-1 | monomers, dimers, trimers, hexamers | Effector molecules impact multimerization, Multiple/protein moonlighting functions, Different assemblies have different activities, pH-dependent oligomeric equilibrium |
| Lactate dehydrogenase | Bacillus stearothermophilus | EC 1.1.1.27 | 9001-60-9 | 2 dimers, tetramer | Effector molecules impact multimerization, Characterized equilibrium of oligomers, Protein concentration dependent specific activity, Mutations shift the equilibrium of oligomers, Oligomer-dependent kinetic parameters, Conformationally distinct oligomeric forms |
| Lon protease | Escherichia coli, Mycobacterium smegmatis | EC 3.4.21.53 | 79818-35-2 | monomer, dimer, trimer, tetramer | Effector molecules impact multimerization, Substrate binding/turnover impacts multimerization, Protein concentration dependent specific activity, Kinetic hysteresis |
| Mitochondrial NAD(P)+ Malic enzyme / [[malate dehydrogenase (oxaloacetate-decarboxylating) (NADP+)]] | Homo sapiens | EC 1.1.1.40 | 9028-47-1 | monomer, 2 dimers, tetramer | Effector molecules impact multimerization, Mutations shift the equilibrium of oligomers, Kinetic hysteresis, |
| Peroxiredoxins | Salmonella typhimurium | EC 1.6.4.- & EC 1.11.1.15 | 207137-51-7 | 2 dimers, decamer | Conformationally distinct oligomeric forms, Different assemblies have different activities |
| Phenylalanine hydroxylase | Homo sapiens | EC 1.14.16.1 | 9029-73-6 | high activity tetramer, low activity tetramer | Substrate binding/turnover impacts multimerization, Conformationally distinct oligomeric forms |
| Phosphoenolpyruvate carboxylase | Escherichia coli, Zea mays | EC [1] | 9067-77-0 | inactive dimer, active tetramer | Effector molecules impact multimerization, Characterized equilibrium of oligomers, Kinetic hysteresis, Conformationally distinct oligomeric forms |
| Phosphofructokinase | Bacillus stearothermophilus, Thermus thermophilus | EC 2.7.1.11 | 9001-80-3 | inactive dimer, active tetramer | Effector molecules impact multimerization, Characterized equilibrium of oligomers |
| Polyphenol oxidase | Agaricus bisporus, Malus domestica, Lactuca sativa L. | EC 1.10.3.1 | 9002-10-2 | monomer, trimer, tetramer, octamer, dodecamer | Multiple/protein moonlighting functions, Substrate binding/turnover impacts multimerization, Different assemblies have different activities, Kinetic hysteresis |
| Porphobilinogen synthase | Drosophila melanogaster, Danio rerio | EC 4.2.1.24 | 9036-37-7 | dimer, hexamer, octamer | PBGS is the prototype morpheein. |
| Pyruvate kinase | Homo sapiens | EC 2.7.1.40 | 9001-59-6 | active and inactive dimers, active tetramer, monomer, trimer, pentamer | Conformationally distinct oligomeric forms |
| Ribonuclease A | Bos taurus | EC 3.1.27.5 3.1.27.5 | 9901-99-4 | monomer, dimer, trimer, tetramer, hexamer, pentamer, higher order | Multiple/protein moonlighting functions, Different assemblies have different activities, Conformationally distinct oligomeric forms |
| Ribonucleotide reductase | Mus musculus | EC 1.17.4.1 | 9047-64-7 | tetramer, hexamer | Effector molecules impact multimerization |
| S-adenosyl-L-homocysteine hydrolase | Dictyostelium discoideum | EC 3.3.1.1 | 9025-54-1 | tetramer and other | Effector molecules impact multimerization |
| Biodegrative threonine dehydratase / threonine ammonia-lyase | Escherichia coli | EC 4.3.1.19 4.3.1.19 | 774231-81-1 | 2 monomers, 2 tetramers | Effector molecules impact multimerization, Characterized equilibrium of oligomers, Different assemblies have different activities |
| β-Tryptase | Homo sapiens | EC 3.4.21.59 | 97501-93-4 | active and inactive monomers, active and inactive tetramers | Protein concentration dependent specific activity, Characterized equilibrium of oligomers |
| Tumor necrosis factor-α | Homo sapiens | 94948-61-5 | monomer, dimer, trimer | Different assemblies have different activities | |
| Uracil phosphoribosyltransferase | Escherichia coli | EC 2.4.2.9 | 9030-24-4 | trimer, pentamer | Effector molecules impact multimerization, Substrate binding/turnover impacts multimerization, Different assemblies have different activities |
