Nasal vaccine
A nasal vaccine is a vaccine administered through the nose that stimulates an immune response without an injection. It induces immunity through the inner surface of the nose, a surface that naturally comes in contact with many airborne microbes. Nasal vaccines are emerging as an alternative to injectable vaccines because they do not use needles and can be introduced through the mucosal route. Nasal vaccines can be delivered through nasal sprays to prevent respiratory infections and diseases like smallpox, Alzheimer’s, influenza, whooping cough, and COVID-19.
History
Nasal inoculation dates as far back as the 17th century in China during the Kangxi Emperor’s reign. Documentation during this period indicates that the Kangxi Emperor vaccinated his family, army, and others for mild smallpox. Manuals detailing vaccination techniques at the time all focused on sending smallpox up the nose of the individual being vaccinated. Although other vaccination techniques were developed using an infected individual’s scabs, a common method was to place a cotton swab with the fluid from an infected person’s pustule up the nose.
Following smallpox, influenza became a prominent focus for nasal vaccine development. The first live attenuated influenza vaccine (LAIV) in the form of a nasal spray was created in Russia by the Institute of Experimental Medicine in 1987. This nasal vaccine development was based on the Russian backbone of LAIV while nasal vaccines since then have been based on other LAIV backbones. The first nasal influenza vaccine was released in the United States in 2001 but was taken off the market due to toxicity concerns. FluMist, one of the most prominent nasal LAIVs, was released in 2003 as nasal LAIVs continued developing.
| Nasal vaccine | |
|---|---|
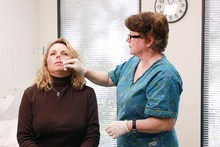
 GI receives an intranasal mist of the flu vaccine at Guantanamo
| |
| Specialty | Vaccine delivery |
Anthrax attacks at the beginning of the 21st century caused a demand for nasal vaccine development. As anthrax is an airborne substance that can be inhaled, a nasal vaccine has the potential to be used to protect individuals from the effects it can have on the respiratory system. Following the September 11, 2001 terrorist attacks in the United States, several individuals at news stations and U.S. senators died after being sent letters with anthrax as an act of bioterrorism. Nasal vaccine research and development against Anthrax was encouraged by the U.S. government in an effort to vaccinate troops.BioThrax, the current anthrax vaccine that is licensed and administered in the United States, requires up to five intramuscular injections and annual boosters; research within the past decade has developed an alternative nasal vaccine that follows the path of infection for anthrax and induces both humoral and cellular immune responses.
The global COVID-19 pandemic led to a rise in nasal vaccines against coronavirus. International efforts for vaccine development occurred as countries such as India, Iran, Russia, and China created nasal COVID-19 vaccines.
Administration
Nasal vaccines are a subsection of mucosal immunization as they use a mucosal route for vaccine delivery. As many pathogens can enter the body through the nose, nasal vaccines take advantage of this mechanism to deliver the vaccine. The nose has multiple lines of defense to prevent pathogens from entering further into the body. Nasal hairs are the first defense as they are at the entrance of the nose and prevent large particles from entering. The mucus layer in the nasal cavity can trap smaller particles that get past the nose hairs. The nasal cavity has a large vascularization network so particles can go through the epithelial layer and directly enter the bloodstream. Intruding particles will interact with the mucosal immune system if they reach the nasal mucosa. The mucosal immune system is composed of lymphoid tissue, B cells, T cells, and antigen-presenting cells. These different types of cells work together to identify intruding particles and trigger an immune response. Nasal vaccines must overcome these barriers and get clearance to deliver the viral antigen to patients. Nasal vaccines must overcome these barriers and get clearance to deliver the viral antigen to patients.
Nasal vaccines can come in different forms such as solutions (liquids), powders, gels, and solid inserts. The most prevalent type of nasal vaccine in research and clinical application is solutions due to its ease of use. Although solutions are usually pipetted into test subjects’ nostrils when conducting animal trials for nasal vaccines, nasal sprays are considered the most practical approach for mass human vaccination using nasal vaccines. A nasal spray is able to bypass the initial layers of the nasal mucosa and deliver the vaccine particles directly to the mucoadhesive layer. The antigen in the nasal vaccine can then trigger an immune response and prevent infection due to nasal vaccines’ accessibility to the immune system.
Nasal sprays are commonly used for delivering drugs in addition to vaccines. Decongestant drugs are often directly delivered to the nose through nasal sprays. Cold and allergy medication can be administered using nasal sprays for local delivery by bypassing nasal hairs and being introduced to the nasal cavity. Intranasal administration can have less drug degradation compared to oral administration because of direct particle delivery. Peptide drugs used for hormone treatments can be delivered nasally through nasal sprays instead of orally to retain particle integrity. Nasal sprays can also be used to deliver diabetes treatment, steroids, and intranasal oxytocin to induce labor. Nasal administration is also used to deliver anesthetics and sedatives due to direct access to the mucosal immune system and bloodstream.
The olfactory epithelium makes up approximately 7% of the surface area of the nasal cavity and is connected to the olfactory bulb in the brain. Drugs and vaccines can be delivered to the brain past the blood-brain barrier through olfactory nerve cells. Vaccines such as Protollin can be used to get past the blood-brain barrier and deliver lipopolysaccharides to the brain to prevent symptoms related to Alzheimer's.
Compared to injectable vaccines, nasal vaccines can be advantageous because they are safe, painless, and easy to use. Nasal vaccines do not require a needle, which eliminates pain from needlestick injuries and safety concerns due to cross-contamination and needle disposal. Some studies also show that intranasal vaccines can generate cross-reactive antibodies that could lead to cross-protection.
Live attenuated influenza vaccine
The live attenuated influenza vaccine (LAIV) in the form of a nasal spray was one of the first nasal vaccines released on the market. Nasal spray LAIVs have been used since the late 1980s as an alternative to the injectable influenza vaccine. Nasal influenza vaccines have become popular as they reduce the risk of intramuscular injuries from administration and are painless. They can also be given more easily to patients because they do not require a needle.
The most prominent nasal LAIV is FluMist, which was released in 2003. FluMist, officially known as FluMist Quadrivalent in the United States and Fluenz in Europe, is known to be the only flu vaccine on the market that does not use a needle. All nasal LAIVs for recent flu seasons (2022-2023) are considered quadrivalents because “they are designed to protect against four types of flu viruses: an influenza A(H1N1) virus, an influenza A(H3N2) virus and two influenza B viruses.” Although injectable and nasal LAIVs are presented as options for yearly vaccination against influenza, FluMist was pulled off of the United States market from 2016 to 2018 due to its inefficiency against a common influenza strain in children. Since then, FluMist has been reformulated and has re-entered the market.
The active ingredients in nasal LAIVs are grown in fertilized chicken eggs. The practice of growing viruses in chicken eggs is common in vaccine production because these viruses need to be grown inside cells. Virus fluid from the incubated chicken eggs is extracted and killed for the viral antigen to be purified for LAIV production. Similar to other vaccines, nasal LAIVs contain ingredients in addition to the viral antigen. Stabilizers such as gelatine, arginine hydrochloride, monosodium glutamate, and sucrose are commonly used in vaccines to assure the vaccines are still effective during and after production, transportation, and storage as well as delivery. Stabilizers are especially important for nasal vaccines as proteases and amino-peptidase in the mucosal membrane can degrade proteins and peptides in vaccines. Research continues to improve nasal LAIVs as influenza affects nearly 9 million people. As influenza changes slightly each year, continuous research on new strains can improve vaccine efficiency. Research on nasal vaccine development for nontypeable Haemophilus influenzae shows that the vaccine binding to surface proteins prevented biofilm formation. As a result, this vaccine can have the potential to treat ear infections caused by biofilm from influenza infection. New components like α-Galactosylceramide (α-GalCer) are also being researched to be used as nasal vaccines against influenza. Since α-GalCer induced immune responses when immunized with a replication-deficient live adenovirus, there is evidence that nasal LAIVs can be co-immunized with other treatments against influenza.
Intranasal COVID-19 Vaccines
Prior to the 2020 global COVID-19 pandemic, animal studies in 2004 on African green monkeys tested a SARS-associated coronavirus (SARS-CoV) vaccine and showed that these monkeys did not emit the virus from their upper respiratory tract after being infected. Since then, several intranasal COVID-19 vaccines have been developed with the onset of the COVID-19 pandemic. inCOVACC, Razi Cov Pars, Sputnik, and Convidicia are nasal COVID-19 vaccines that were developed throughout the world to improve vaccine availability and reduce the spread of COVID-19.
In August 2020, during the COVID-19 pandemic, studies in mice and monkeys demonstrated that protection from the new coronavirus might be obtained through the nasal route. Another study postulated that if a COVID-19 vaccine could be given by a spray in the nose, people might be able to vaccinate themselves. Research about the main characteristics of nasal spray vaccines that can affect the efficiency of vaccine delivery for COVID-19 indicates that the spray cone angle can impact the delivery efficiency; droplet initial velocity and composition did not have as much of an impact on nasal vaccine efficiency as the spray cone angle.
India and China approved inCOVACC and Convidecia, respectively, to be used as boosters for those who have already received at least two COVID-19 vaccine doses. Although nasal COVID-19 vaccine research continues in the United States, lack of government funding could prevent this research from moving on to human trials to get approval for public administration. Privately funded research for nasal COVID-19 vaccines is starting to reach clinical trials; a nasal COVID-19 vaccine by Blue Lake Biotechnology has started its Phase 1 clinical trials as of late February 2023. Scientists speculate that nasal vaccines might have an advantage over other types of vaccines because they provide immune defense at the site of administration.
Applications to Veterinary Medicine
Species other than humans use nasal vaccines to prevent diseases. Intranasal vaccines are used on dogs for Bordetella bronchiseptica to prevent infectious tracheobronchitis (ITB). ITB, commonly known as kennel cough, typically spreads in highly populated environments such as kennels and dog shelters. Consistent vaccination against ITB using an intranasal vaccine can create an immune response to protect the vaccinated dog. Consistent vaccination against ITB using an intranasal vaccine can create an immune response to protect the vaccinated dog.
Cattle receive nasal vaccines against diseases such as bovine herpesvirus 1, parainfluenza type 3, and bovine rhinotracheitis virus. As all three of these viruses are related to respiratory infection, using an intranasal route can bring the vaccine directly to the respiratory system.
Recent discoveries indicate that rainbow trout have a previously unknown lymphoid structure in their nasal cavity. This structure allows them to have fast innate and adaptive responses to nasal vaccines.
Current Research on Nasal Vaccines
Current research is exploring new technologies and developments to improve nasal vaccine delivery methods. Particle size and characteristics have become a focus of research as smaller particles can travel more easily to reach the epithelial layer of the nasal cavity compared to larger particles. Nanoparticles and nanosystems are being researched to optimize nasal delivery. Coated nanoparticles are an area of focus due to their properties to induce immune effects. Glycol chitosan-coated nanoparticles induced more of an immune response compared to the other types of nanoparticles. Nanocarriers designed based on the characteristics of the nasal epithelium can be used to deliver nasal vaccines and can therefore make nasal vaccination more accessible. Polymeric nanosystems are also being developed to deliver vaccines to target sites while preventing them from degrading; current research is focused on understanding the material and physical properties of biodegradable materials to be used in nanosystems to improve vaccine efficacy. Research on the movement of nasal vaccine particles is focused on developing more effective ways for these vaccines to enter the body. An animal study on mice tested how a nasal vaccine can bypass issues with entry into the nasal epithelium by taking advantage of ciliary movement. The results indicated that tubulin tyrosine ligase-like family member 1 (Ttll1) knockout mice had higher levels of the vaccine antigen compared to the hetero mice.
In addition to nasal sprays, nanogels are a current area of focus for nasal vaccine research. One study created a cationic nanogel-based nasal vaccine that bound to surface proteins in the nasal cavity of mice. This binding prevented biofilm formation, which subsequently allowed for the treatment of ear infections. Another recent study focused on testing a previously developed nanogel nasal vaccine to prevent pneumococcal infection in macaques. The conclusions from this study showed that macaques that received this nasal vaccine had reduced lung inflammation and bacteria in the lungs.
Newer research expands beyond LAIV and COVID-19. A single-dose nasal vaccine using live attenuated Bordetella pertussis, the bacteria that causes whooping cough, was tested on young mice and compared to mice injected with an acellular vaccine. The nasal vaccine was able to prevent symptoms related to whooping cough and have effects comparable to injectable vaccines. Although smallpox has been almost completely eliminated due to vaccination, there are still some cases throughout the world that warrant continuous development of smallpox vaccines. There is still research being done in the 21st century to prevent smallpox from reoccurring; a 2008 study analyzed vaccinia virus (VACV) mutants when administered intranasally to mice. The findings from this study showed that low doses of intranasal vaccinations protected the mice and therefore could be used as vectors for vaccines against smallpox and similar viruses.
Studies are also being conducted on humans to understand the clinical translation of nasal vaccine developments. The importance of nasal spray nozzle distance and particle distribution characteristics has been shown in a 2023 study on infants. The results indicated that the minimum efficiency of the four sprays tested in vitro was 80% and the spray droplet size distribution and distance between the spray nozzle and nose can affect the vaccine’s delivery. Another important characteristic of the nasal spray nozzle is the spray cone angle. A study on a 6-year-old patient to prevent COVID-19 through nasal vaccination showed that the spray cone angle can affect vaccine delivery efficiency while droplet initial velocity and composition do not have as much of an impact.