
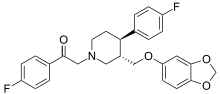
Omiloxetine
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H25F2NO4 |
| Molar mass | 465.497 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.3±0.1 g/cm3 |
| Melting point | 228.65 °C (443.57 °F) |
| Boiling point | 587.2 °C (1,089.0 °F) |
| Solubility in water | 0.0015 mg/mL (20 °C) |
| |
| |
Omiloxetine (omiloextinum, omiloxetino INN) was a selective serotonin reuptake inhibitor drug candidate that underwent preclinical development by the Spanish pharmaceutical company, Ferrer Internacional, until 2005, when it was abandoned.
Rafael Foguet also patented Abaperidone.
| |||||||||||||||||||||
| |||||||||||||||||||||
|
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
|
DAT (DRIs) |
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
NET (NRIs) |
|
||||||||||||||
|
SERT (SRIs) |
|
||||||||||||||
| VMATs | |||||||||||||||
| Others |
|
||||||||||||||