
Peroxynitrite
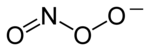 Chemical structure of the peroxynitrite anion
| |
| Names | |
|---|---|
|
IUPAC name
Oxido nitrite
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NO3− | |
| Molar mass | 62.005 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−. It is a structural isomer of nitrate, NO−
3
Preparation
Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide:
- NO + O−2 → NO(O2)−
It is prepared by the reaction of hydrogen peroxide with nitrite:
- H2O2 + NO−
2 → ONOO− + H2O
Its presence is indicated by the absorbance at 302 nm (pH 12, ε302 = 1670 M−1 cm−1).
Reactions
Peroxynitrite is weakly basic with a pKa of ~6.8.
It is reactive toward DNA and proteins.
ONOO− reacts nucleophilically with carbon dioxide. In vivo, the concentration of carbon dioxide is about 1 mM, and its reaction with ONOO− occurs quickly. Thus, under physiological conditions, the reaction of ONOO− with carbon dioxide to form nitrosoperoxycarbonate (ONOOCO−
2) is by far the predominant pathway for ONOO−. ONOOCO−
2 homolyzes to form carbonate radical and nitrogen dioxide, again as a pair of caged radicals. Approximately 66% of the time, these two radicals recombine to form carbon dioxide and nitrate. The other 33% of the time, these two radicals escape the solvent cage and become free radicals. It is these radicals (carbonate radical and nitrogen dioxide) that are believed to cause peroxynitrite-related cellular damage.
Peroxynitrous acid
Its conjugate acid peroxynitrous acid is highly reactive, although peroxynitrite is stable in basic solutions.
See also
|
Nitrogen species
| |
|---|---|
| Hydrides | |
| Organic | |
| Oxides | |
| Halides | |
| Oxidation states | |
