Potassium in biology
Potassium is the main intracellular ion for all types of cells, while having a major role in maintenance of fluid and electrolyte balance. Potassium is necessary for the function of all living cells, and is thus present in all plant and animal tissues. It is found in especially high concentrations within plant cells, and in a mixed diet, it is most highly concentrated in fruits. The high concentration of potassium in plants, associated with comparatively very low amounts of sodium there, historically resulted in potassium first being isolated from the ashes of plants (potash), which in turn gave the element its modern name. The high concentration of potassium in plants means that heavy crop production rapidly depletes soils of potassium, and agricultural fertilizers consume 93% of the potassium chemical production of the modern world economy.
The functions of potassium and sodium in living organisms are quite different. Animals, in particular, employ sodium and potassium differentially to generate electrical potentials in animal cells, especially in nervous tissue. Potassium depletion in animals, including humans, results in various neurological dysfunctions. Characteristic concentrations of potassium in model organisms are: 30–300mM in E. coli, 300mM in budding yeast, 100mM in mammalian cell and 4mM in blood plasma.
Function in plants
The main role of potassium in plants is to provide the ionic environment for metabolic processes in the cytosol, and as such functions as a regulator of various processes including growth regulation. Plants require potassium ions (K+) for protein synthesis and for the opening and closing of stomata, which is regulated by proton pumps to make surrounding guard cells either turgid or flaccid. A deficiency of potassium ions can impair a plant's ability to maintain these processes. Potassium also functions in other physiological processes such as photosynthesis, protein synthesis, activation of some enzymes, phloem solute transport of photoassimilates into source organs, and maintenance of cation:anion balance in the cytosol and vacuole.
Function in animals
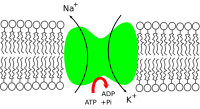
Potassium is the major cation (K+, a positive ion) inside animal cells, while sodium (Na+) is the major cation outside animal cells. The difference between the concentrations of these charged particles causes a difference in electric potential between the inside and outside of cells, known as the membrane potential. The balance between potassium and sodium is maintained by ion transporters in the cell membrane. All potassium ion channels are tetramers with several conserved secondary structural elements. A number of potassium channel structures have been solved including voltage gated,ligand gated,tandem-pore, and inwardly rectifying channels, from prokaryotes and eukaryotes. The cell membrane potential created by potassium and sodium ions allows the cell to generate an action potential—a "spike" of electrical discharge. The ability of cells to produce electrical discharge is critical for body functions such as neurotransmission, muscle contraction, and heart function.
Dietary recommendations
The U.S. National Academy of Medicine (NAM), formerly known as the Institute of Medicine, sets Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs), or Adequate Intakes (AIs) for when there is not sufficient information to set EARs and RDAs. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes. The current AI for potassium for women and men ages 14 and up is 4700 mg. AI for pregnancy equals 4700 mg/day. AI for lactation equals 5100 mg/day. For infants 0–6 months 400 mg, 6–12 months 700 mg, 1–13 years increasing from 3000 to 4500 mg/day. As for safety, the NAM also sets Tolerable upper intake levels (ULs) for vitamins and minerals, but for potassium the evidence was insufficient, so no UL established.
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For people ages 15 and older the AI is set at 3,500 mg/day. AIs for pregnancy is 3,500 mg/day, for lactation 4,000 mg/day. For children ages 1–14 years the AIs increase with age from 800 to 2,700 mg/day. These AIs are lower than the U.S. RDAs. The EFSA reviewed the same safety question and decided that there was insufficient data to establish a UL for potassium.
Labeling
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For potassium labeling purposes 100% of the Daily Value was 3500 mg, but as of May 2016, it has been revised to 4700 mg. A table of the old and new adult Daily Values is provided at Reference Daily Intake.
Supplements
20 mEq (781 mg) potassium from potassium gluconate (4680 mg), or potassium citrate (2040 mg), mixed a half-cup (1.12 dL) water, taken two to four times a day, may be used on daily basis.
Labeling
Because of the risk of small-bowel lesions, the US FDA requires some potassium salts (for example potassium chloride) containing more than 99 mg (about 1.3 mEq) to be labeled with a warning.
Food sources
Eating a variety of foods that contain potassium is the best way to get an adequate amount. Foods with high sources of potassium include kiwifruit, orange juice, potatoes, coconut, avocados, apricots, parsnips and turnips, although many other fruits, vegetables, legumes, and meats contain potassium.
Common foods very high in potassium:
- beans (white beans and others), dark leafy greens (spinach, Swiss chard, and others), baked potatoes, dried fruit (apricots, peaches, prunes, raisins; figs and dates), baked squash, yogurt, fish (salmon), and avocado;
- nuts (pistachios, almonds, walnuts, etc.) and seeds (squash, pumpkin, sunflower)
The most concentrated foods (per 100 grams) are:
- dried herbs, sun dried tomatoes, cocoa solids, whey powder, paprika, yeast extract, rice bran, molasses, and dry roasted soybeans
Deficiency
High blood pressure/Hypertension
Diets low in potassium increase risk of hypertension, stroke and cardiovascular disease.
Hypokalemia
A severe shortage of potassium in body fluids may cause a potentially fatal condition known as hypokalemia. Hypokalemia typically results from loss of potassium through diarrhea, diuresis, or vomiting. Symptoms are related to alterations in membrane potential and cellular metabolism. Symptoms include muscle weakness and cramps, paralytic ileus, ECG abnormalities, intestinal paralysis, decreased reflex response and (in severe cases) respiratory paralysis, alkalosis and arrhythmia.
In rare cases, habitual consumption of large amounts of black licorice has resulted in hypokalemia. Licorice contains a compound (Glycyrrhizin) that increases urinary excretion of potassium.
Insufficient intake
Adult women in the United States consume on average half the AI, for men two-thirds. For all adults, fewer than 5% exceed the AI. Similarly, in the European Union, insufficient potassium intake is widespread.
Side effects and toxicity
Gastrointestinal symptoms are the most common side effects of potassium supplements, including nausea, vomiting, abdominal discomfort, and diarrhea. Taking potassium with meals or taking a microencapsulated form of potassium may reduce gastrointestinal side effects.
Hyperkalemia is the most serious adverse reaction to potassium. Hyperkalemia occurs when potassium builds up faster than the kidneys can remove it. It is most common in individuals with renal failure. Symptoms of hyperkalemia may include tingling of the hands and feet, muscular weakness, and temporary paralysis. The most serious complication of hyperkalemia is the development of an abnormal heart rhythm (arrhythmia), which can lead to cardiac arrest.
Although hyperkalemia is rare in healthy individuals, oral doses greater than 18 grams taken at one time in individuals not accustomed to high intakes can lead to hyperkalemia.
See also
- Biology and pharmacology of chemical elements
- Action potential – Neuron communication by electric impulses
- Calcium in biology – Use of calcium by organisms
- Electrolyte – Ionic solids whose dissociation in water free up ions carrying the electrical current in solution
- Iodine in biology – Use of Iodine by organisms
- Magnesium in biology – Use of Magnesium by organisms
- Membrane potential – Type of physical quantity
- Selenium in biology – Use of Selenium by organisms
- Sodium in biology – Use of Sodium by organisms
Further reading
- "Potassium Health Professional Fact Sheet". NIH Office of Dietary Supplements. 3 April 2020.
External links
- "Potassium". Drug Information Portal. U.S. National Library of Medicine.
- Brooks/Cole publishers – Sodium Potassium pump
- Oregon State University – Micronutrient Information Center
- Potassium at Lab Tests Online
- Potassium: analyte monograph - the Association for Clinical Biochemistry and Laboratory Medicine.
|
Clinical biochemistry blood tests
| |||||
|---|---|---|---|---|---|
| Electrolytes | |||||
| Acid-base | |||||
| Iron tests | |||||
| Hormones | |||||
| Metabolism | |||||
| Cardiovascular | |||||
| Liver function tests | |||||
| Pancreas | |||||
| Small molecules |
|
||||
| Proteins |
|
||||