
Reproterol
Подписчиков: 0, рейтинг: 0
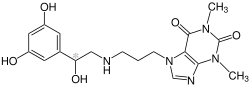 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Inhalation (MDI), IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.579 |
| Chemical and physical data | |
| Formula | C18H23N5O5 |
| Molar mass | 389.412 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Reproterol is a short-actingβ2 adrenoreceptor agonist used in the treatment of asthma.
It was patented in 1965 and came into medical use in 1977.
Stereochemistry
Reproterol contains a stereocenter and is chiral. There are thus two enantiomers, the (R)-form and the (S)-form. The commercial preparations contain the drug as a racemate, an equal mixture of the two enantiomers.
| Enantiomers of reproterol | |
|---|---|
 (R)-Reproterol CAS number: 210710-33-1 |
 (S)-Reproterol CAS number: 210710-34-2 |
| α1 |
|
||||
|---|---|---|---|---|---|
| α2 |
|
||||
| β |
|
||||