
Rociletinib
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| Trade names | Xegafri |
| Other names | CO-1686, AVL-301 |
| Routes of administration |
By mouth |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
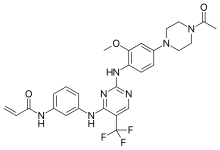
| Formula | C27H28F3N7O3 |
| Molar mass | 555.562 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rociletinib is a medication developed to treat non-small cell lung carcinomas with a specific mutation. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor. It was being developed by Clovis Oncology as a potential treatment for non-small-cell lung cancer. In May 2016, development of rociletinib was halted, along with its associated clinical trials, and Clovis Oncology withdrew its marketing authorisation application from the European Medicines Agency.
| Angiopoietin |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CNTF |
|
||||||||||
| EGF (ErbB) |
|
||||||||||
| FGF |
|
||||||||||
| HGF (c-Met) |
|
||||||||||
| IGF |
|
||||||||||
| LNGF (p75NTR) |
|
||||||||||
| PDGF |
|
||||||||||
| RET (GFL) |
|
||||||||||
| SCF (c-Kit) |
|
||||||||||
| TGFβ |
|
||||||||||
| Trk |
|
||||||||||
| VEGF |
|
||||||||||
| Others |
|
||||||||||