Sarecycline
 |
|
| Pronunciation |
sar"e sye' kleen |
| Trade names |
Seysara |
| Other names |
P-005672 |
|
AHFS/Drugs.com
|
Monograph |
| MedlinePlus |
a618068 |
| License data |
|
Routes of
administration |
By mouth |
| ATC code |
|
|
| Legal status |
|
|
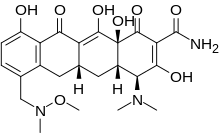
(4S,4aS,5aR,12aR)-4-(Dimethylamino)-1,10,11,12a-tetrahydroxy-7-[[methoxy(methyl)amino]methyl]-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide
|
| CAS Number |
|
|
PubChem CID
|
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| PDB ligand |
|
| ECHA InfoCard |
100.241.852
|
|
| Formula |
C24H29N3O8
|
| Molar mass |
487.509 g·mol−1
|
| 3D model (JSmol) |
|
CN(C)[C@H]1[C@@H]2C[C@@H]3CC4=C(C=CC(=C4C(=C3C(=O)[C@@]2(C(=C(C1=O)C(=O)N)O)O)O)O)CN(C)OC
|
Sarecycline, sold under the brand name Seysara , is a narrow-spectrum tetracycline-derived antibiotic medication. It is specifically designed for the treatment of acne, and was approved by the FDA in October 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older. Two randomized and well-controlled clinical trials reported efficacy data on both facial and truncal acne (back and chest). Efficacy was assessed in a total of 2002 subjects 9 years of age and older. Unlike other tetracycline-class antibiotics, sarecycline has a long C7 moiety that extends into and directly interact with the bacterial messenger RNA (mRNA). The spectrum of activity is limited to clinically relevant Gram-positive bacteria, mainly Cutibacterium acnes, with little or no activity against Gram-negative bacterial microflora commonly found in the human gastrointestinal tract.
Medical uses
Sarecycline, is indicated for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris.

