
Sitravatinib
Подписчиков: 0, рейтинг: 0
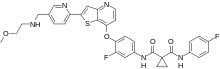 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C33H29F2N5O4S |
| Molar mass | 629.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sitravatinib (MGCD516) is an experimental drug for the treatment of cancer. It is a small molecule inhibitor of multiple tyrosine kinases.
Sitravatinib is being developed by Mirati Therapeutics.
Ongoing phase II trials include a trial for liposcarcoma, a combination trial for non-small cell lung cancer, and a combination trial with nivolumab for renal cell carcinoma. Sitravatinib is being evaluated in ongoing trials in patients with advanced non-small cell lung cancer, including in a combination trial with nivolumab in those who are resistant to checkpoint inhibitor therapy, and certain patients who are naïve to checkpoint inhibitor therapy.