
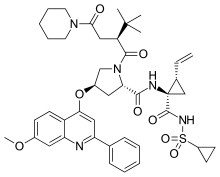
Sovaprevir
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| Other names | ACH-1625 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C43H53N5O8S |
| Molar mass | 799.98 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sovaprevir (codenamed ACH-1625) is an experimental drug designed to treat the hepatitis C virus. It is under development by Achillion Pharmaceuticals. It acts as a NS3/4A inhibitor.Sovaprevir received fast track status from the U.S. Food and Drug Administration in 2012.
External links
| Hepatitis C |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hepatitis D | |||||||||
| Picornavirus | |||||||||
| Anti-influenza agents | |||||||||
| Multiple/general |
|
||||||||
| |||||||||