
Temoporfin
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.152.970 |
| Chemical and physical data | |
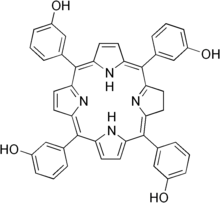
| Formula | C44H32N4O4 |
| Molar mass | 680.764 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Temoporfin (INN) is a photosensitizer (based on chlorin) used in photodynamic therapy for the treatment of squamous cell carcinoma of the head and neck . It is marketed in the European Union under the brand name Foscan. The U.S. Food and Drug Administration (FDA) declined to approve Foscan in 2000. The EU approved its use in June 2001.
Good results were obtained in 21 of 35 patients treated in Germany.
It is photoactivated at 652 nm i.e. by red light.
Patients can remain photosensitive for several weeks after treatment.