
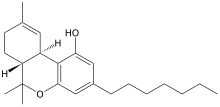
Tetrahydrocannabiphorol
 | |
 | |
| Clinical data | |
|---|---|
| Other names | (-)-Trans-Δ9-tetrahydrocannabiphorol Δ9-THCP (C7)-Δ9-THC THC-Heptyl |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H34O2 |
| Molar mass | 342.523 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity approximately 33 times that of Delta-9-THC.
Isomers
Delta-3-THCP
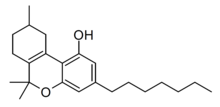
The Δ3/Δ6a(10a) isomer Δ3-THCP was synthesised in 1941, and was found to have around the same potency as Δ3-THC, unlike the hexyl homologue parahexyl which was significantly stronger.
Delta-8-THCP

The Δ8 isomer is also known as a synthetic cannabinoid under the code name JWH-091, It's unconfirmed whether or not Delta-8-THCP is found naturally in cannabis plants, but likely is due to Delta-8-THC itself being a degraded form of Delta-9-THC. JWH-091 has approximately double the binding affinity at the CB1 receptor (22nM ± 3.9nM) in comparison to Delta-9-THC (40.7nM ± 1.7nM) or Delta-8-THC (44nM ± 12nM). but appears significantly lower in vitro than the binding activity of Delta-9-THCP (Ki = 1.2 nM CB1)
See also
- CBD-DMH
- Delta-8-THC
- Hexahydrocannabinol
- HU-210
- JWH-138
- Parahexyl
- Perrottetinene
- Tetrahydrocannabivarin
- Tetrahydrocannabutol
- Tetrahydrocannabihexol
- O-1871
- DMHP
- Cannabicyclohexanol