
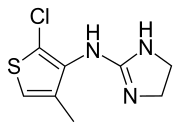
Tiamenidine
 | |
| Clinical data | |
|---|---|
| Trade names | Sundralen, Symcorad, Symcor |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 2.3–5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C8H10ClN3S |
| Molar mass | 215.70 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Tiamenidine (BAN, USAN, INN, also known as thiamenidine, Hoe 440) is an imidazoline compound that shares many of the pharmacological properties of clonidine. It is a centrally-acting α2 adrenergic receptor agonist (IC50 = 9.1 nM). It also acts as an α1-adrenergic receptor agonist to a far lesser extent (IC50 = 4.85 μM). In hypertensive volunteers, like clonidine, it significantly increased sinus node recovery time and lowered cardiac output. It was marketed (as tiamenidine hydrochloride) by Sanofi-Aventis under the brand name Sundralen for the management of essential hypertension.
Synthesis
Reaction of thiourea 1 with methyl iodide gives the corresponding S-methyl analogue (2), followed by heating with ethylenediamine, completes the synthesis of tiamenidine (3).
See also
|
Sympatholytics (antagonize α-adrenergic vasoconstriction) |
|||||
|---|---|---|---|---|---|
| Other antagonists |
|
||||
| |||||
| α1 |
|
||||
|---|---|---|---|---|---|
| α2 |
|
||||
| β |
|
||||
