
Vonoprazan
Vonoprazan
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| Trade names | Takecab, Vocinti |
| License data |
|
| Drug class | Potassium-competitive acid blocker |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 80% |
| Metabolism | Liver, by cytochrome P450 (3A4, 2B6, 2C19, 2D6) |
| Elimination half-life | 7.7 h |
| Duration of action | > 24 h |
| Excretion | Kidney |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
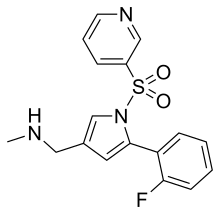
| Formula | C17H16FN3O2S |
| Molar mass | 345.39 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vonoprazan, sold under the brand name Takecab among others, is a first-in-class potassium-competitive acid blocker medication. It was approved in the Japanese market in February 2015 and in Russia in April 2021.
Vonoprazan is used in form of the fumarate for the treatment of gastroduodenal ulcer (including some drug-induced peptic ulcers) and reflux esophagitis, and can be combined with antibiotics for the eradication of Helicobacter pylori.
Co-packaged combinations of vonoprazan with amoxicillin and vonoprazan with amoxicillin and clarithromycin were approved for medical use in the United States in May 2022.
Society and culture
Names
Vonoprazan is the international nonproprietary name (INN).
External links
- "Vonoprazan". Drug Information Portal. U.S. National Library of Medicine.
| H2 antagonists ("-tidine") | |
|---|---|
|
Prostaglandins (E)/ analogues ("-prost-") |
|
|
Proton-pump inhibitors ("-prazole") |
|
|
Potassium-competitive acid blockers ("-prazan") |
|
| Others | |
| Combinations | |
| |