
WIN 55,212-2
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
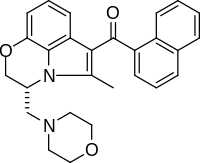
| Formula | C27H26N2O3 |
| Molar mass | 426.516 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |

WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.
WIN 55,212-2 is a potent cannabinoid receptor agonist that has been found to be a potent analgesic in a rat model of neuropathic pain. It activates p42 and p44 MAP kinase via receptor-mediated signaling.
At 5 μM WIN 55,212-2 inhibits ATP production in sperm in a CB1 receptor-dependent fashion.
WIN 55,212-2, along with HU-210 and JWH-133, may prevent the inflammation caused by amyloid beta proteins involved in Alzheimer's disease, in addition to preventing cognitive impairment and loss of neuronal markers. This anti-inflammatory action is induced through agonist action at cannabinoid receptors, which prevents microglial activation that elicits the inflammation.
WIN 55,212-2 is a full agonist at the CB1 cannabinoid receptor (Ki = 1.9 nM) and has much higher affinity than THC (Ki = 41 nM) for this receptor. WIN 55,212-2 is also an agonist of the PPARα and PPARγ nuclear receptors.
WIN 55,212-2 reduces voluntary wheel running in laboratory mice, but with effects that depend on both genetic background and sex.
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as WIN 55,212-2 are Schedule I Controlled Substances. WIN 55,212-2 is illegal in the UK.
See also
- WIN 48,098 (Pravadoline)
- WIN 54,461 (6-Bromopravadoline)
- WIN 55,225 (JWH-200)
- WIN 56,098
Further reading
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM (July 2006). "The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin". Proceedings of the National Academy of Sciences of the United States of America. 103 (30): 11393–8. Bibcode:2006PNAS..10311393P. doi:10.1073/pnas.0603861103. PMC 1544096. PMID 16849427.
- Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML (February 2005). "Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation". The Journal of Neuroscience. 25 (8): 1904–13. doi:10.1523/JNEUROSCI.4540-04.2005. PMC 6726060. PMID 15728830.
External links
- "Win 55,212-2 Data Sheet". Enzo Life Sciences.
|
Psychedelics (5-HT2A agonists) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Dissociatives (NMDAR antagonists) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Deliriants (mAChR antagonists) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Receptor |
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Transporter |
|
||||||||||||
|
Enzyme |
|
||||||||||||
| Others |
|
||||||||||||
| |||||||||||||
| TRPA |
|
||||
|---|---|---|---|---|---|
| TRPC |
|
||||
| TRPM |
|
||||
| TRPML |
|
||||
| TRPP |
|
||||
| TRPV |
|
||||
See also: Receptor/signaling modulators • Ion channel modulators | |||||