
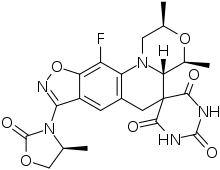
Zoliflodacin
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| Other names | AZD0914; ETX0914 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| Drug class | Antibiotic |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 97.8% |
| Metabolism | Hepatic |
| Onset of action |
|
| Elimination half-life | 5.3–6.3 h |
| Excretion | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C22H22FN5O7 |
| Molar mass | 487.444 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zoliflodacin (development codes AZD0914 and ETX0914) is an experimental antibiotic that is being studied for the treatment of infection with Neisseria gonorrhoeae (gonorrhea). It has a novel mechanism of action which involves inhibition of bacterial type II topoisomerases. It is being developed by Entasis Therapeutics and is (as of 2020) in Phase III clinical trials.