
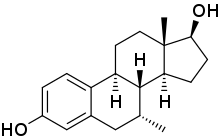
7α-Methylestradiol
Подписчиков: 0, рейтинг: 0
Not to be confused with Methylestradiol.
 | |
| Clinical data | |
|---|---|
| Other names | 7α-Methyl-E2; 7α-Me-E2; 7α-Methylestra-1,3,5(10)-triene-3,17β-diol |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H26O2 |
| Molar mass | 286.415 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
7α-Methylestradiol (7α-Me-E2), also known as 7α-methylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrogen and an active metabolite of the androgen/anabolic steroid trestolone. It is considered to be responsible for the estrogenic activity of trestolone. The compound shows about the same affinity for the estrogen receptor as estradiol.
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | |
|---|---|---|---|---|---|---|---|---|
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7 | <0.1 | |
| 7α-Methylestradiol | 1–3 | 15–25 | 101 | <1 | <1 | ? | ? | |
| Trestolone | 50–75 | 100–125 | ? | <1 | ? | ? | ? | |
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. | ||||||||