
Etynodiol
 | |
| Clinical data | |
|---|---|
| Other names | Ethynodiol; 3β-Hydroxynorethisterone; 17α-Ethynylestr-4-ene-3β,17β-diol |
| Drug class | Progestin; Progestogen |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.013.610 |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etynodiol, or ethynodiol, is a steroidal progestin of the 19-nortestosterone group which was never marketed. A diacylated derivative, etynodiol diacetate, is used as a hormonal contraceptive.Etynodiol is sometimes used as a synonym for etynodiol diacetate.
It was patented in 1955.
Pharmacology
Etynodiol is a prodrug of norethisterone, and is converted immediately and completely into norethisterone. Etynodiol is an intermediate in the conversion of the prodrug lynestrenol into norethisterone.
| Compound | Typea | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisterone | Metabolite | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisterone | Metabolite | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | Metabolite | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
| Norethisterone acetate | Prodrug | 20 | 5 | 1 | 0 | 0 | ? | ? |
| Norethisterone enanthate | Prodrug | ? | ? | ? | ? | ? | ? | ? |
| Noretynodrel | Prodrug | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Etynodiol | Prodrug | 1 | 0 | 11–18 | 0 | ? | ? | ? |
| Etynodiol diacetate | Prodrug | 1 | 0 | 0 | 0 | 0 | ? | ? |
| Lynestrenol | Prodrug | 1 | 1 | 3 | 0 | 0 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Footnotes: a = Active or inactive metabolite, prodrug, or neither of norethisterone. Sources: See template. | ||||||||
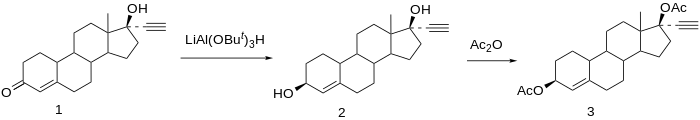
Chemistry
Etynodiol is a 19-nortestosterone derivative. Structurally, it is almost identical to norethisterone and lynestrenol, differing only in its C3 substituent. Whereas norethisterone has a ketone at C3 and lynestrenol has no substituent at C3, etynodiol has a hydroxyl group at the position.
Synthesis
Society and culture
Generic names
Etynodiol is the generic name of the drug and its INN, while ethynodiol is its BAN.
| ER |
|
||||||
|---|---|---|---|---|---|---|---|
| GPER |
|
||||||
| AR |
|
||||||
|---|---|---|---|---|---|---|---|
| GPRC6A |
|
||||||