
Fluphenazine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prolixin, Modecate, Moditen others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682172 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth, Intramuscular injection, depot injection (fluphenazine decanoate) |
| Drug class | Typical antipsychotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 2.7% (by mouth) |
| Metabolism | unclear |
| Elimination half-life | IM 15 hours (HCL), 7–10 days (decanoate) |
| Excretion | Urine, feces |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.639 |
| Chemical and physical data | |
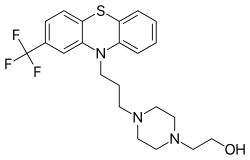
| Formula | C22H26F3N3OS |
| Molar mass | 437.53 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fluphenazine, sold under the brand name Prolixin among others, is a high-potency typical antipsychotic medication. It is used in the treatment of chronic psychoses such as schizophrenia, and appears to be about equal in effectiveness to low-potency antipsychotics like chlorpromazine. It is given by mouth, injection into a muscle, or just under the skin. There is also a long acting injectable version that may last for up to four weeks. Fluphenazine decanoate, the depot injection form of fluphenazine, should not be used by people with severe depression.
Common side effects include movement problems, sleepiness, depression and increased weight. Serious side effects may include neuroleptic malignant syndrome, low white blood cell levels, and the potentially permanent movement disorder tardive dyskinesia. In older people with psychosis as a result of dementia it may increase the risk of dying. It may also increase prolactin levels which may result in milk production, enlarged breasts in males, impotence, and the absence of menstrual periods. It is unclear if it is safe for use in pregnancy.
Fluphenazine is a typical antipsychotic of the phenothiazine class. Its mechanism of action is not entirely clear but believed to be related to its ability to block dopamine receptors. In up to 40% of those on long term phenothiazines, liver function tests become mildly abnormal.
Fluphenazine came into use in 1959. The injectable form is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. It was discontinued in Australia in 2017.
Medical use
A 2018 Cochrane review found that fluphenazine was an imperfect treatment and other inexpensive drugs less associated with side effects may be an equally effective choice for people with schizophrenia.
Side effects
Discontinuation
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse. Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite. Other symptoms may include restlessness, increased sweating, and trouble sleeping. Less commonly there may be a feeling of the world spinning, numbness, or muscle pains. Symptoms generally resolve after a short period of time.
There is tentative evidence that discontinuation of antipsychotics can result in psychosis. It may also result in reoccurrence of the condition that is being treated. Rarely tardive dyskinesia can occur when the medication is stopped.
Pharmacology
Pharmacodynamics
Fluphenazine acts primarily by blocking post-synaptic D2 receptors in the basal ganglia, cortical and limbic system. It also blocks alpha-1 adrenergic receptors, muscarinic-1 receptors, and histamine-1 receptors.
| Site | Ki (nM) | Action | Ref |
|---|---|---|---|
| 5-HT1A | 145-2829 | ND | |
| 5-HT1B | 334 | ND | |
| 5-HT1D | 334 | ND | |
| 5-HT1E | 540 | ND | |
| 5-HT2A | 3.8-98 | ND | |
| 5-HT2B | ND | ND | |
| 5-HT2C | 174–2,570 | ND | |
| 5-HT3 | 4,265- > 10,000 | ND | |
| 5-HT5A | 145 | ND | |
| 5-HT6 | 7.9 - 38 | ND | |
| 5-HT7 | 8 | ND | |
| D1 | 14.45 | ND | |
| D2 | 0.89 | ND | |
| D2L | ND | ||
| D3 | 1.412 | ND | |
| D4 | 89.12 | ND | |
| D5 | 95–2,590 | ND | |
| α1A | 6.4-9 | ND | |
| α1B | 13 | ND | |
| α2A | 304-314 | ND | |
| α2B | 181.6-320 | ND | |
| α2C | 28.8-122 | ND | |
| β1 | > 10,000 | ND | |
| β2 | > 10,000 | ND | |
| H1 | 7.3-70 | ND | |
| H2 | 560 | ND | |
| H3 | 1,000 | ND | |
| H4 | > 10,000 | ND | |
| M1 | 1,095-3,235.93 | ND | |
| M2 | 2,187.76-7,163 | ND | |
| M3 | 1441–1445.4 | ND | |
| M4 | 5,321 | ND | |
| M5 | 357 | ND | |
| SERT | ND | ND | |
| NET | ND | ND | |
| DAT | ND | ND | |
| NMDA (PCP) |
ND | ND | |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. All data are for human cloned proteins, except 5-HT3 (rat), D4 (human/rat), H3 (guinea pig), and NMDA/PCP (rat). | |||
Pharmacokinetics
| Medication | Brand name | Class | Vehicle | Dosage | Tmax | t1/2 single | t1/2 multiple | logPc | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole lauroxil | Aristada | Atypical | Watera | 441–1064 mg/4–8 weeks | 24–35 days | ? | 54–57 days | 7.9–10.0 | |
| Aripiprazole monohydrate | Abilify Maintena | Atypical | Watera | 300–400 mg/4 weeks | 7 days | ? | 30–47 days | 4.9–5.2 | |
| Bromperidol decanoate | Impromen Decanoas | Typical | Sesame oil | 40–300 mg/4 weeks | 3–9 days | ? | 21–25 days | 7.9 | |
| Clopentixol decanoate | Sordinol Depot | Typical | Viscoleob | 50–600 mg/1–4 weeks | 4–7 days | ? | 19 days | 9.0 | |
| Flupentixol decanoate | Depixol | Typical | Viscoleob | 10–200 mg/2–4 weeks | 4–10 days | 8 days | 17 days | 7.2–9.2 | |
| Fluphenazine decanoate | Prolixin Decanoate | Typical | Sesame oil | 12.5–100 mg/2–5 weeks | 1–2 days | 1–10 days | 14–100 days | 7.2–9.0 | |
| Fluphenazine enanthate | Prolixin Enanthate | Typical | Sesame oil | 12.5–100 mg/1–4 weeks | 2–3 days | 4 days | ? | 6.4–7.4 | |
| Fluspirilene | Imap, Redeptin | Typical | Watera | 2–12 mg/1 week | 1–8 days | 7 days | ? | 5.2–5.8 | |
| Haloperidol decanoate | Haldol Decanoate | Typical | Sesame oil | 20–400 mg/2–4 weeks | 3–9 days | 18–21 days | 7.2–7.9 | ||
| Olanzapine pamoate | Zyprexa Relprevv | Atypical | Watera | 150–405 mg/2–4 weeks | 7 days | ? | 30 days | – | |
| Oxyprothepin decanoate | Meclopin | Typical | ? | ? | ? | ? | ? | 8.5–8.7 | |
| Paliperidone palmitate | Invega Sustenna | Atypical | Watera | 39–819 mg/4–12 weeks | 13–33 days | 25–139 days | ? | 8.1–10.1 | |
| Perphenazine decanoate | Trilafon Dekanoat | Typical | Sesame oil | 50–200 mg/2–4 weeks | ? | ? | 27 days | 8.9 | |
| Perphenazine enanthate | Trilafon Enanthate | Typical | Sesame oil | 25–200 mg/2 weeks | 2–3 days | ? | 4–7 days | 6.4–7.2 | |
| Pipotiazine palmitate | Piportil Longum | Typical | Viscoleob | 25–400 mg/4 weeks | 9–10 days | ? | 14–21 days | 8.5–11.6 | |
| Pipotiazine undecylenate | Piportil Medium | Typical | Sesame oil | 100–200 mg/2 weeks | ? | ? | ? | 8.4 | |
| Risperidone | Risperdal Consta | Atypical | Microspheres | 12.5–75 mg/2 weeks | 21 days | ? | 3–6 days | – | |
| Zuclopentixol acetate | Clopixol Acuphase | Typical | Viscoleob | 50–200 mg/1–3 days | 1–2 days | 1–2 days | 4.7–4.9 | ||
| Zuclopentixol decanoate | Clopixol Depot | Typical | Viscoleob | 50–800 mg/2–4 weeks | 4–9 days | ? | 11–21 days | 7.5–9.0 | |
| Note: All by intramuscular injection. Footnotes: a = Microcrystalline or nanocrystalline aqueous suspension. b = Low-viscosity vegetable oil (specifically fractionated coconut oil with medium-chain triglycerides). c = Predicted, from PubChem and DrugBank. Sources: Main: See template. | |||||||||
History
Fluphenazine came into use in 1959.
Availability
The injectable form is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. It was discontinued in Australia in 2017.
Veterinary
In horses, it is sometimes given by injection as an anxiety-relieving medication, though there are many negative common side effects and it is forbidden by many equestrian competition organizations.
External links
- "Fluphenazine". Drug Information Portal. U.S. National Library of Medicine.
| Classes | |
|---|---|
|
Antidepressants (Tricyclic antidepressants ) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics | |
| Others |
|
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood agents |
|
||||||||||||||
| Blister agents |
|
||||||||||||||
| Nerve agents |
|
||||||||||||||
| Neurotoxins |
|
||||||||||||||
| Nettle agents |
|
||||||||||||||
| Pulmonary/ choking agents |
|
||||||||||||||
| Vomiting agents | |||||||||||||||
| Incapacitating agents |
|
||||||||||||||
| Lachrymatory agents |
|
||||||||||||||
| Malodorant agents | |||||||||||||||
| Cornea-clouding agents | |||||||||||||||
| Biological toxins | |||||||||||||||
| Other |
|
||||||||||||||