
Gaboxadol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.059.039 |
| Chemical and physical data | |
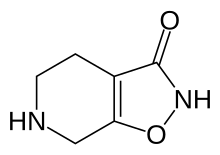
| Formula | C6H8N2O2 |
| Molar mass | 140.142 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Gaboxadol, also known as 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP), is a conformationally constrained derivative of the alkaloid muscimol that was first synthesized in 1977 by the Danish chemist Povl Krogsgaard-Larsen. In the early 1980s gaboxadol was the subject of a series of pilot studies that tested its efficacy as an analgesic and anxiolytic, as well as a treatment for tardive dyskinesia, Huntington's disease, Alzheimer's disease, and spasticity. It was not until 1996 that researchers attempted to harness gaboxadol's frequently reported sedative "adverse effect" for the treatment of insomnia, resulting in a series of clinical trials sponsored by Lundbeck and Merck. In March, 2007, Merck and Lundbeck cancelled work on the drug, citing safety concerns and the failure of an efficacy trial. It acts on the GABA system, but in a different way from benzodiazepines, Z-Drugs, and barbiturates. Lundbeck states that gaboxadol also increases deep sleep (stage 4). Unlike benzodiazepines, THIP does not demonstrate reinforcement in mice or baboons despite activation of dopaminergic neurons in the ventral tegmental area.
In 2015, Lundbeck sold its rights to the molecule to Ovid Therapeutics, whose plan is to develop it for FXS and Angelman syndrome. It is known internally in Ovid as OV101.
See also
External links
- 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- H. Lundbeck Website
- Medical News Today article
- Report of cancellation of development.
- Gaboxadol
| Ionotropic |
|
||||
|---|---|---|---|---|---|
| Metabotropic |
|
||||
|
Receptor (ligands) |
|
||||
|---|---|---|---|---|---|
|
Transporter (blockers) |
|
||||