
Penfluridol
 | |
| Clinical data | |
|---|---|
| Trade names | Semap |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.689 |
| Chemical and physical data | |
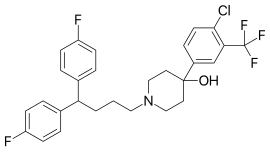
| Formula | C28H27ClF5NO |
| Molar mass | 523.965 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Penfluridol (Semap, Micefal, Longoperidol) is a highly potent, first generation diphenylbutylpiperidine antipsychotic. It was discovered at Janssen Pharmaceutica in 1968. Related to other diphenylbutylpiperidine antipsychotics, pimozide and fluspirilene, penfluridol has an extremely long elimination half-life and its effects last for many days after single oral dose. Its antipsychotic potency, in terms of dose needed to produce comparable effects, is similar to both haloperidol and pimozide. It is only slightly sedative, but often causes extrapyramidal side-effects, such as akathisia, dyskinesiae and pseudo-Parkinsonism. Penfluridol is indicated for antipsychotic treatment of chronic schizophrenia and similar psychotic disorders, it is, however, like most typical antipsychotics, being increasingly replaced by the atypical antipsychotics. Due to its extremely long-lasting effects, it is often prescribed to be taken orally as tablets only once a week (q 7 days). The once-weekly dose is usually 10–60 mg. A 2006 systematic review examined the use of penfluridol for people with schizophrenia:
| Summary | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Although there are shortcomings and gaps in the data, there appears to be enough overall consistency for different outcomes. The effectiveness and adverse effects profile of penfluridol are similar to other typical antipsychotics; both oral and depot. Furthermore, penfluridol is shown to be an adequate treatment option for people with schizophrenia, especially those who do not respond to oral medication on a daily basis and do not adapt well to depot drugs. One of the results favouring penfluridol was a lower drop out rate in medium term when compared to depot medications. It is also an option for people with long-term schizophrenia with residual psychotic symptoms who nevertheless need continuous use of antipsychotic medication. An additional benefit of penfluridol is that it is a low-cost intervention. | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
Synthesis

The Grignard reaction between Methyl 4-oxopiperidine-1-carboxylate [29976-54-3] (1) and 5-bromo-2-chlorobenzotrifluoride [445-01-2] (2) gives methyl 4-[4-chloro-3-(trifluoromethyl)phenyl]-4-hydroxypiperidine-1-carboxylate, CID:134990265 (3). Deprotection of the urethane gives 4-[4-Chloro-3-(trifluoromethyl)phenyl]-4-piperidinol [21928-50-7] (4). Lastly alkylation with 1,1'-(4-Chlorobutylidene)bis(4-fluorobenzene) [3312-04-7] (5) completed the synthesis of Penfluridol (6).
See also
- Typical antipsychotic
- Diphenylbutylpiperidine
- Fluspirilene
- Pimozide
- Carpipramine (atypical antipsychotic)
- Clocapramine (atypical antipsychotic)
Further reading
- Benkert O, Hippius H (1976). Psychiatrische Pharmakotherapie (2nd ed.). Springer-Verlag. ISBN 3-540-07916-5.
| D1-like |
|
||||||
|---|---|---|---|---|---|---|---|
| D2-like |
|
||||||
| H1 |
|
||||
|---|---|---|---|---|---|
| H2 |
|
||||
| H3 |
|
||||
| H4 |
|
||||